
Mortality and Cancer Risks, Which Pills to Avoid & Better Alternatives

Mortality and Cancer Risks, Which Pills to Avoid & Better Alternatives
By Daniel F. Kripke, M.D.

Sleeping Pills Could Shorten Your Life
WARNING: Sleeping pills are hazardous to your health and could cause death from cancer, infections, overdoses, respiratory failure, other illnesses or accidents.
For over 40 years, as a doctor and medical researcher, I have worked to assess the risks of sleeping pills. I have learned that sleeping pills are associated with significantly increased mortality. Sleeping pills kill people.
This means that people who take sleeping pills die sooner than people who do not use sleeping pills. On average, those taking sleeping pills die several years early.
Chapter 1 describes how sleeping pills cause cancer, illnesses, and deaths. Chapter 2 describes how sleeping pills fail to improve sleep substantially, and on average make function worse the next day.
I first became interested when I saw the work of Dr. E. Cuyler Hammond at the American Cancer Society. In 1975, I went to visit The American Cancer Society, starting a collaboration which lasted for many years. American Cancer Society data from over one million people showed that use of sleeping pills was associated with more deaths within six years, but insomnia by itself was not associated with any death risk.
As of July, 2018, there were at least 42 published studies of the mortality risks of sleeping pills. Of the 42 studies which reported either greater or lesser mortality associated with sleeping pills, 40 studies showed that people taking sleeping pills died sooner. (The last two studies were a mix of evidence that sleeping pills caused deaths in some ways and other evidence they did not.) For more medical details about mortality and other risks of sleeping pills, follow the link to a comprehensive review [1], and be sure to check updates.
Here is an example of these studies. From electronic medical records,[2] we studied over 10,000 patients who took sleeping pills and over 20,000 matched patients who did not take sleeping pills. The patients
who took sleeping pills died 4.6 times as often during follow-ups averaging 2.5
years. Patients who took higher doses (averaging over 132 pills per year) died
5.3 times as often. Even those patients who took fewer than 18 pills per year
had 3.6 times the deaths of patients who took no hypnotics. Other newer and even larger studies have more recently reported similar results.
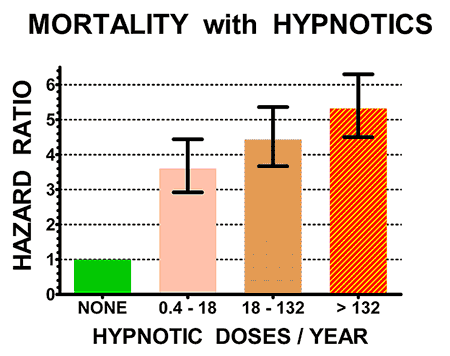
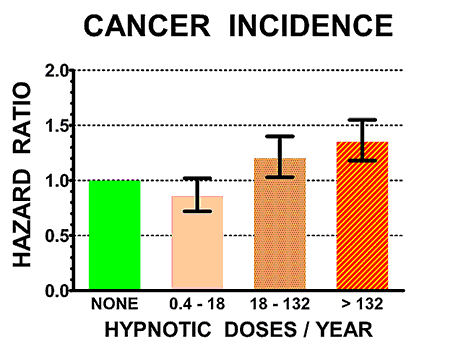
The illustrations above show the hazard ratios for mortality (above) and cancer incidence (below) for the control non-users of hypnotics (doses/year = NONE, in green) and for three groups of users of hypnotics with increasing numbers of doses/year prescribed. Hazard ratios above 1.0 are estimates of how many times the mortality rates or cancer incidence of sleeping-pill users exceeded that of controls. The heavy black bars show the statistical 95% confidence limits of the hazard estimates, that is, statistically the hazard ratio of the sample is 95% likely to be within the bars above and below the vertical black lines. However, unknown biases in the samples could produce true risks outside the confidence limits.
Patients who took sleeping pills died 4.6 times more often (on average) than patients who avoided sleeping pills.
Notice that people who took 18 pills a year or less (most no more than three pills) had substantially higher mortality. Our study and several more recent studies indicated that even one or two prescriptions might be lethal. The risk was especially high for people taking opioid pain pills, because sleeping pills increase the narcotic overdose risk. Sleeping pills are especially dangerous combined with narcotics, alcohol or both.
It seems quite likely that the sleeping pills were causing early death for many of the patients. In addition, those who averaged two to three sleeping pills per week or more were 35% more likely to develop a new cancer.
We made great efforts to match the patients taking sleeping pills with those not taking sleeping pills for age, sex, smoking history, and various measures of poor health, so it seemed to be a fair comparison. Nevertheless, it is true that finding that sleeping pill use is associated with early death does not by itself prove that the sleeping pills are causing those deaths. Theoretically, there could be confounding factors or biases in the selection of patients which caused those deaths without involving sleeping pills. We can only say that we found almost no evidence of such biases. Although there was certainly at least a small amount of confounding, it seemed to us unlikely that biases could entirely explain all of those excess deaths and cancers.
If sleeping pills cause even a small portion of the excess deaths and cancers associated with their use, they are too dangerous to use.
Some readers will remember when the cigarette companies claimed that the fact that cigarette smoking is associated with cancer and early death did not prove that cigarettes cause cancer. Cigarette manufacturers have by now given up on that argument. The risks are quite similar with sleeping pills. For absolute proof, we would need large randomized controlled trials of cigarettes or sleeping pills, but nobody is going to do such trials that now would be hard to make ethical. If the cigarette or sleeping pill companies believed that such trials would prove that their products were safe, they would have done such controlled trials many years ago.
The specific sleeping pills we studied were zolpidem (e.g., Ambien), temazepam (e.g., Restoril), eszopiclone (e.g., Lunesta), zaleplon (e.g., Sonata), other benzodiazepines such as triazolam (e.g., Halcion) and flurazepam (e.g., Dalmane), barbiturates, and sedative antihistamines such as diphenhydramine (e.g., Benadryl). Most of the patients in this study were taking zolpidem or temazepam. We had less data about the other drugs. However, all of the sleeping pills studied were significantly associated with excess mortality. Because of the way the study was done and its limited size, we could not say that one sleeping pill was safer than another.
Sleeping
Pills Associated with Significant Mortality Risk
Zolpidem
Temazepam
Eszopiclone
Zaleplon
Triazolam
Flurazepam
Estazolam
Quazepam
Barbiturates
(esp. phenobarbital)
Antihistamines, mainly diphenhydramine
These results do not necessarily apply to any sleeping pill which was not included in our study, except perhaps zopiclone (because zopiclone is half eszopiclone). Zopiclone is a sleeping pill popular outside the United States. Specifically, the risk data did not cover doxepin, ramelteon, melatonin, suvorexant, or trazodone, though some of those might also be unsafe.[3]
1.A. New sleeping pills cause cancer in animals
Were the epidemiologic studies just statistical accidents, or do sleeping pills really cause cancer? Several years ago, the Food and Drug Administration (FDA) started making available on the internet some of their documents about the review of those newer sleeping pills approved for marketing in the United States since 1998. You can find these documents yourself through the US Food & Drug Administration’s Online Service, Drugs@FDA.[4]
To my great surprise, I learned that rats and mice given high doses of zaleplon (Sonata), eszopiclone (Lunesta) as part of zopiclone, and ramelteon (Rozerem) developed cancer. The information available was a little vague to be certain, but it seems that these new sleeping pills all caused cancer in animals. FDA reviewers thought some of the results were worrisome. One of the reasons I am not sure I understand these results is that I cannot find that the companies have ever published the study details in the medical literature. It is conceivable that the manufacturers do not want these cancer experiments understood. Some of the drugs also broke chromosomes, which is a well-known specific chemical mechanism by which drugs cause cancer. The FDA has large animal testing facilities but has not seemed interested in checking if sleeping pills cause cancer.
There was also some older and confusing information about zolpidem (Ambien). Although one of the old records[5] seemed to say that animals given zolpidem developed three kinds of cancer, and FDA reviewers were concerned about the risks, the new labeling approved[6] for the extended release version of zolpidem (Ambien CR) says no evidence of carcinogenic potential was observed in either mice or rats. I would like to know how the company figured they do not owe people a warning. Some FDA scientists also wondered, according to internal documents I found.
1.B. Evidence that sleeping pills cause cancer in people
In 2005 and 2006, several new sleeping pills were introduced into the U.S. market. The industry was hoping to make several billion dollars a year. Because the companies wanted to market sleeping pills for long-term consumption, they did larger studies of long-term use than ever had been done before. Summaries of the data from these randomizing controlled trials can be found at the FDA internet site[7] for zaleplon (Sonata), eszopiclone (Lunesta), and ramelteon (Rozerem). It turned out that because zaleplon was compared to zolpidem as well as to placebo, there was a bit of zolpidem data available also.
I admit that it is hard to understand the details of these controlled trials from the data which FDA has made available, but fortunately, I persuaded the FDA to review their own files. According to the FDA, there were nine new skin cancers and four other cancers among study participants randomized to the sleeping pills, but zero new cancers among those who only received placebo. The best estimate would be that the cancer rate for participants randomized to sleeping pills was several times that of the luckier volunteers who received placebo. Because these data come from randomizing comparisons, they appear to be proof that new sleeping pills (as a group) cause cancer. However, the controlled trial data were not sufficient to prove that any specific sleeping pill or brand causes cancer.[8] Let’s put together the epidemiologic data, the animal data, and the data from combining these controlled trials for four drugs. The evidence is that a patient who takes any of the sleeping pills listed in the box above is increasing his or her risk of getting cancer. I feel that my patients should be warned about this risk.
We do not have clear evidence that one sleeping pill has more cancer risk than another. In our epidemiologic study, we only demonstrated statistically significant cancer risks specifically for zolpidem and temazepam, the most popular drugs in that study, but none of the other drugs for which we had less data were clearly any better or worse. For patients prescribed over 2-3 sleeping pills per week, there was a 35% increased risk of developing cancer within an average of 2.5 years.
A new study from Taiwan has appeared, based on a representative national health insurance data base.[9] These authors studied zolpidem, which was the most popular hypnotic in Taiwan and the United States. With over eight years of follow-up, the Taiwan authors found a considerably larger cancer hazard associated with zolpidem than our study found with shorter follow-up. There have been additional studies with similar results. [10]
1.C. More about lethal risks of sleeping pills
As a young medical student in my first year of training, one of the first things I learned in our student laboratory was that the humane way to “put an animal to sleep” was to administer a fatal dose of a barbiturate such as pentobarbital. A bit later, I learned that pentobarbital was being prescribed almost automatically as a sleeping pill for patients in the hospital. Pentobarbital and related drugs are currently used to execute the death penalty on prisoners. Any medical student knows that these drugs can kill.
Doctors have a wonderfully complete understanding of how sleeping pills such as pentobarbital kill animals. These drugs bind with protein molecules called GABA receptors on the surface of nerve cells. The same protein receptor molecules bind at the same time with a neurotransmitter chemical called GABA, which gives them their name. Barbiturates and other sleeping pills accentuate the action of GABA, which is to cause the receptor molecule to allow chloride ions to enter the nerve cells. Since the chloride ions are negatively charged, they make the inside the nerve cell more negatively polarized, which in turn, makes the nerve cells less likely to fire (to generate nerve activity). When the nerve cells which stimulate the muscles of breathing are overly inhibited from firing by sleeping pills, the animal stops breathing. When breathing stops, the animal dies within a few minutes from lack of oxygen. This same mechanism explains how sleeping pills kill people who take an overdose. Mixture with other drugs, particularly opioids, alcohol and other sedatives, greatly magnifies the risks, as do various medical conditions, possibly stopping breathing with a dose not intended to be lethal.
In the 1970’s, a new group of sleeping pills became popular, molecules which chemically are named benzodiazepines. The first sold as tranquilizers were chlordiazepoxide (Librium) and diazepam (Valium). Soon, the benzodiazepine flurazepam (Dalmane) was marketed as a sleeping pill, and flurazepam soon dominated the market. The main advantage of benzodiazepines is that they initially seemed less likely to produce acute overdose deaths than barbiturates.[11]
A third generation of new sleeping pills have been benzodiazepine agonists, which means that the chemical molecules may not have the benzodiazepine structure of drugs like Valium, but they act at the same brain receptors. Epidemiologic data have not confirmed that benzodiazepines are safer than barbiturates, perhaps because of how they are combined with other drugs and alcohol.
There is an age-old belief that sleeping pills might help depressed patients. Rather, sleeping pill manufacturers’ controlled trials proved that sleeping pills can cause depression.[12] In fact, the sleeping pills examined in one study seemed to double the rate of new depressions. Use of sleeping pills is very strongly associated with suicide from all causes.
Suicide, accidental overdose and cancer are probably not the most common ways in which sleeping pills kill, but the other ways are more poorly understood and less well documented. Here are some of the other possible mechanisms.
All approved sleeping pills can cause “hangover,” that is, they not only reduce the action potentials of our brain cells during sleep, but they can also reduce brain cell activity during the day.[13] This can make us sleepy, less alert, confused, and weak during the day. We will discuss psychological consequences of this hangover in the next chapter, but here the issue is impairments of survival. Falls are much more common among elderly people who are taking hypnotics.[14] Of patients given Lunesta, 10% had accidents as compared to 6% given placebo in one study, and falls were specifically more common with Lunesta.[15] Because several studies show that people who are responsible for automobile accidents are unusually likely to have sleeping pills in their blood[16], it is thought that hangover may often cause automobile accidents, as well as other fatal accidents. The publicity about Ambien zombies driving like sleep walkers provides some extremely vivid examples.[17]
In the last 20 years, physicians have become concerned about sleep apnea, a condition where there are pauses of breathing during sleep. Physicians suspect that sleep apnea can cause deaths during sleep. Not all studies agree, but several studies have found that when a person with sleep apnea takes sleeping pills, there are more pauses in breathing and the pauses last longer. I was surprised to learn in the FDA data how well-documented it is that zolpidem makes sleep apnea worse. Because sleeping pills risk making apnea worse, many experts recommend that people with apnea should not be given sleeping pills. The problem is that almost everybody above age 40 has some sleep apnea, and the majority of people over 65 would meet commonly-used criteria for a diagnosis of sleep apnea.[18] Therefore, a large proportion of people taking sleeping pills are making their apnea worse. Over a period of many years, anything which makes sleep apnea worse would be expected to cause high blood pressure, and therefore, to increase the risk of heart attacks, heart failure, and strokes.
A final concern regarding mortality is how people care for themselves. Because sleeping pills, like tranquilizers, reduce worry about possible threats and risks in our lives, it is possible that the hangover effects of sleeping pills would reduce people’s attentiveness in taking care of themselves.

Other Risks of Sleeping Pills
2.A. Sleeping pills impair daytime thinking.
The side effects of the prescription sleeping pills are much like their benefits. At night, we want our brain cells to stop working, so sleeping pills make brain cells less active. If the sleeping pill is in the blood during the day, it will make the daytime brain less active and less functional. The problem is that no sleeping pill remains in the blood all night, impairing consciousness, and then suddenly evaporates at the moment of awakening. Besides, many people who take sleeping pills do get up at night, at a time when the sleeping pill could cause falls or confusion. Most of the marketed prescription hypnotics, when taken at bedtime, will remain in the blood with at least half strength when morning comes.
Only a few prescription hypnotics marketed in the U.S. leave the blood fast enough to be largely gone from the blood by morning: these include zolpidem (Ambien), zaleplon (Sonata), and triazolam (Halcion). Even these drugs may be found in the morning blood if they are taken in the middle of the night, and even some patients taking Ambien at bedtime were found to act like drunk drivers early in the morning. This risk may be worse among women, who metabolize Ambien more slowly. Ambien CR is more likely than ordinary Ambien to affect people the next morning, and eszopiclone (Lunesta) is likely to produce a few hours of morning impairment, particularly among people over age 60.
On January 10, 2013, the FDA issued a warning recommending that the usual dose of zolpidem (Ambien) be no more than 6.25 mg for women or elders of any gender. The FDA had finally realized that a dangerous percentage of patients have enough zolpidem in the blood the next morning to impair performance such as driving. Because (as the Ambien manufacturer has admitted), the now-recommended lower doses are largely “ineffective” for sleep, most people use higher doses with higher risks that the FDA now regards as unsafe. There simply is no dosage level that is generally both safe and effective. The same seems to be true of eszopiclone, zopiclone, and some of the other sleeping pills that have been studied less completely.
Oddly enough, despite the brief half-life (time to be half-dissipated) of zolpidem, zaleplon, and triazolam, there is fragmentary evidence that short-acting hypnotics can produce impairments lasting after their expected disappearance from the blood.[19] Perhaps this is because a percentage of people have genetic variations in their metabolism of sleeping pills which may cause dangerous concentrations to linger. Ramelteon (Rozerem) produces no next-day impairment according to the manufacturer studies, but one well-controlled independent European study showed impairment in driving performance.
As explained above, sleeping pills suppress the action potentials of a wide variety of brain cells. The psychological effects are to make us sleepy, reduce alertness and vigilance, slow reaction times and judgment, and impair aspects of intelligence and memory. Literally hundreds of studies have been done concerning the psychological effects of sleeping pills, both within a few hours after ingestion and then during the day following taking a sleeping pill at bedtime.[20] To summarize an extremely complex group of studies, almost all sleeping pills produce immediate impairments of memory and performance. Further, there is extensive evidence that sleeping pills impair performance and memory on the following day.
Sleeping pills generally make function WORSE the next day.
To view sleeping pill advertising, you might imagine sleeping pills help you to work better, think better, or function better the next day. This is deceptive. With very few exceptions, controlled studies supported by the manufacturers showed that sleeping pills made test performance WORSE on the following day, or else had no definite effect on performance. Look through the FDA files for Ambien, Lunesta, Sonata, Rozerem, Belsomra, or Silenor at the FDA website.[21] See if you can find any evidence that these drugs improved next-day performance for people with insomnia. You will not find any. For the older sleeping pills, there are less definite data available in FDA files, but many studies of older hypnotics showed that sleeping pills impaired performance the next day.
The problem of daytime impairment is more severe with the longer-acting drugs such as flurazepam (Dalmane) and quazepam (Doral), because the active by-products of these drugs remain in the blood for days following only a single dose. When one of the long-acting drugs is taken every night, the blood concentrations accumulate day by day, increasing for up to 10-20 days, reaching much higher concentrations than after the initial dose. Therefore, with flurazepam (Dalmane) and quazepam (Doral), and also with diazepam (Valium) and chlordiazepoxide (Librium) when they are taken nightly as sleeping pills, daytime impairment accumulates after consecutive days of use.[22] Because the build-up of these drugs in blood happens slowly, patients may not realize that their intelligence, reflexes, and judgment are slowly fading, and relatives may not know the cause.[23]
The insomnia sufferer’s hope and belief that a prescription sleeping pill will improve function on the next day are consistently betrayed. It simply does not work. Insomniacs taking sleeping pills are like alcoholics claiming that alcohol improves their driving: everybody seems to realize that sedatives impair driving except the drunk driver.
To repeat, as a generalization, taking sleeping pills at bedtime impairs how people perform on the following day.[24] There might be a few studies suggesting minor exceptions, but these studies were not very relevant to insomnia patients treated with prescription hypnotics in the United States.
2.B. A telling study.
Some years ago, I was privileged to participate with a group of sleep experts from different medical schools in a study sponsored by Hoffmann-La Roche, the makers of Dalmane (flurazepam). Because Dalmane impairs driving, the manufacturer wanted to see if a very-short-acting benzodiazepine would improve performance. The short-acting drug tested was midazolam, which is sold as a hypnotic in Europe, though in the U.S. it is marketed only as a short-acting anesthetic. Many experiments on hypnotic effects on performance had used young healthy volunteers, who had little room for improvement in their sleep. We thought that healthy volunteers might benefit less than insomniacs who really had disturbed sleep. Therefore, we recruited a group of chronic insomniacs who said they had had insomnia and had taken benzodiazepines successfully for an average of over 13 years.[25] Moreover, we selected volunteers in whom we could verify with EEG-sleep recording that their sleep really was disturbed at night, and then we withdrew these people from their sleeping pills for at least 4 weeks. Once withdrawn from whatever they had been taking, they were studied for two baseline nights while receiving a placebo pill. Then, the volunteers were randomly assigned to receive Dalmane, to receive midazolam, or to continue receiving inactive placebo pills.
As expected, these chronic insomniacs slept about 20-27 min. more for the first two nights they were given Dalmane or midazolam than those given the placebo.[26] That was not much improvement. Remarkably, by nine or 14 days of administration, there were no statistically-reliable increases at all in the sleep of the volunteers taking Dalmane or midazolam as compared to those receiving placebo. The volunteers had become tolerant to the sleeping pills, which had lost their benefit. Part of the reason that the sleeping pills showed no significant benefit after 14 days was that the placebo group had improved. Perhaps regular sleep habits and the belief that they were being helped had produced this placebo improvement, and possibly, placebo patients improved because they had been two weeks longer off the benzodiazepines they had been previously taking, which might have been making them worse. This is an important point, because the fact that a person taking a sleeping pills is sleeping more than during an experimental baseline does not mean that the pill is helping, a point confused in many of the most-quoted studies that do not emphasize a parallel contrast with randomized placebo. In any case, after two weeks, the groups receiving Dalmane and midazolam were not significantly improved compared to placebo patients.
Our hope that these powerful hypnotics would increase sleep in these chronic insomniacs (for even two weeks) was disappointed.
The small increase in sleep which Dalmane and midazolam produced on the first two nights of administration was too small to produce any improvement in performance, which was measured the following mornings with a variety of sophisticated testing methods. Moreover, by 14 days, both drugs were making performance significantly worse. On tests reflecting driving performance, these sleeping pills would have made the patients less safe drivers. This study shocked the author with an important lesson: the sleeping pills made the patients worse, not better.
This study yielded another important observation in these chronic insomniacs who for years had believed in sleeping pills. The volunteers themselves said that they thought the research sleeping pills were good and were helping them, even when objective tests and at times, their own family, observed that the hypnotics were making them worse. Even the group receiving placebo said that placebo was a good sleeping pill which they would like to use again. That is a lesson in the misperception of sleeping pill users. These patients were self-deceived about the value of the medication, almost deluded, thinking the medicines made them better which actually made them worse. Users of addicting sleeping pills are like heroin addicts: they may claim they need the drug, but to medical people and their own families, it looks like the addicting drugs are very harmful.
A rather similar study of chronic insomniacs receiving flurazepam (Dalmane) or triazolam (Halcion) also showed that after several weeks of use, the drugs were no better than placebo.[27] This study was interesting because it studied the period of withdrawal after the research drugs were stopped. Even though the volunteers receiving triazolam slept no better than those given placebo at the end of five weeks, when the drugs were stopped, those who had received triazolam developed a drug-withdrawal insomnia which made them worse than those who had taken placebo. This study implied that after several weeks of use, people may take sleeping pills not because they continue to benefit in any way, but because their sleep becomes so much worse when they withdraw. It hurts too much to stop. In effect, they have become dependent on sleeping pills or addicted.
These two studies were important because they were focused on the kinds of people who were actually long-term users of sleeping pills. The studies showed that sleeping pills produced no long-term benefits, only harms. Also, the studies showed that the volunteers thought they were benefitting from the drugs (even placebo), even when they were being harmed.
Another more recent study looked at intermittent use of zolpidem (Ambien) three times a week. After several weeks of use, those taking this sleeping pill were sleeping better on nights when they took the drug as compared to placebo but then worse when they skipped it.[28] Overall, after several weeks of use, the group taking Ambien was averaging no better sleep than the randomly-selected group taking inactive placebo, despite the dependence and withdrawal symptoms to which they were subjecting themselves.
A very important study emphasized the greater benefit of cognitive-behavioral therapy than zolpidem. Two groups of insomnia patients were randomized to both start treatment with cognitive therapy combined with zolpidem, but then one group discontinued zolpidem and the other group was permitted to take zolpidem as needed. Patients who continued to take zolpidem as-needed after two years slept worse than patients who tapered off zolpidem. [29] I would interpret these studies as indicating that continuing use of zolpidem made insomnia worse.
The manufacturers now admit that both zolpidem (Ambien) and eszopiclone (Lunesta) cause withdrawal insomnia on the night after you stop the pill. Anxiety may also occur as a withdrawal symptom. People become addicted to these drugs because they experience such anxiety and poor sleep, whenever they try to stop. If they stayed off the drug for a few days, they might sleep just as well without the medication. Even worse, newer studies indicate that with sleeping pills, drug-withdrawal damage may be surprisingly lasting. Indeed, we are not certain if the damage ever completely heals.
2.C. Disastrous side effects.
We now realize that sleeping pills can cause some very peculiar and disastrous psychological side effects. Because sleeping pills turn off our brain cells – not always in all parts of the brain to an equal extent – they can make people do some mighty peculiar things. For example, having taken Ambien, people can act like somnambulists or sleep walkers or robots gone haywire. In the more amusing examples, they may sleep-walk to the refrigerator and stuff themselves with strange foods that they would not normally eat in such quantity. Of course, this is not amusing if it leads to obesity, which can be a life-threatening condition, or if they eat something unhealthy. The behavior of the so-called Ambien Zombies is not always amusing. In a few reported cases, people intoxicated with Ambien have climbed into their cars and engaged in sleep driving. Some had serious accidents.[30] Hallucinations have been reported with zolpidem, zaleplon, and eszopiclone.[31] At other times, people receiving sleeping pills have become confused or disoriented. Another odd symptom is complete amnesia for events, even during the day. For example, a successful businessman told me that while taking Ambien at night, he might have absolutely no recollection of a conference which his own notes showed that he had attended the following day. From viewing various reports, I now realize that these terrible side effects may develop in about one percent of users of sleeping pills.
I do not think that these strange symptoms are unique to the new non-benzodiazepine hypnotics such as zolpidem, though in recent years, Ambien was getting most of the bad publicity. Similar lapses in memory and strange behaviors were reported frequently when triazolam was the most popular sleeping pill.[32] A lawyer once asked me to consult with her client in the jail, where he was awaiting trial for having murdered his sister. The lawyer said her client thought that the Halcion (triazolam) he had been taking had caused him to commit this irrational crime, because otherwise he had no idea why he had done it. There would be no way of knowing for certain if Halcion was the explanation, but I wouldn’t be surprised if the murderer had been a Halcion Zombie. One wonders if these reports have been most common with Halcion and Ambien because they were the market leaders, but it is interesting that both drugs are absorbed and removed from blood at about the same speed. I am inclined to think that these disastrous side effects are not so uncommon and probably can occur with most prescription sleeping pills.
Another side effect of sleeping pills is depression. The sleeping pill industry would like to emphasize that insomnia leads to depression, which might be true some of the time. They imply that sleeping pills can prevent depression. That is not so. The controlled trials of zaleplon, zolpidem, eszopiclone, and ramelteon submitted to the FDA along with some published studies showed a higher rate of developing depression among those given the sleeping pills as compared to those given placebo. This proved that sleeping pills caused people to have more depression. Perhaps a common mechanism is that insomnia leads to sleeping pill use, which in turn leads to depression. Multiple studies have found that sleeping pill use is associated with very high suicide rates, but as yet, the evidence that sleeping pills cause increased suicide is based on the strong evidence that the pills cause depression, as well as very high rates of suicide observed among those known to have taken sleeping pills. Likewise, higher rates of depression and higher rates of overdose death are observed among patients receiving zolpidem in addition to opioid pain medications. Belsomra (suvorexant), although its biochemical mechanisms are different from other sleeping pills, also seems to cause suicidal thinking.
2.D. Lollipops, not sleeping pills.
The motivations of physicians to give patients sleeping pills have not been studied extensively, but there is some interesting evidence. Physicians are supposed to explain their medical thinking in their medical records. Even in the medical records of a distinguished teaching hospital, not one of 331 charts of patients receiving sleeping pills had a proper record of why the pill was given.[33] It is safe to assume that there often was no good medical justification. It has been the same in the hospitals where I taught. In the hospital, however, the staff motivations are not hard to understand.
Everyone has heard the stories of nurses awakening patients to give them sleeping pills. When I was a medical student, I learned that nurses want to keep their patients quiet for the night. Physicians routinely write sleeping pill orders in the hospital without specific medical goals, because they hate for nurses to call at night and wake the doctor up to get a sleeping pill order. As a medical student, I was instructed that if I wanted to sleep at night, I had better routinely prescribe a sleeping pill for every patient. The sleeping pill was to help the doctors and nurses sleep, not for the patients. Moreover, there is no evidence that sleeping pills really help hospital personnel at night, since sleeping pills seem to produce problems like falls and delirium that do not make the work of the night staff easier.
When I was a child, my pediatrician would give me a lollipop at every visit to compensate for the pain of needles I bravely faced. Doctors don’t give lollipops to adult patients, but sleeping pills are sometimes prescribed the same way. Giving sleeping pills is often a gift-giving behavior which is part of the “bedside manner.” When a distinguished group of physicians from our national Institute of Medicine were asked in which situations they would give a patient a sleeping pill, they said it was when they knew the patient well. The decision had to do with the doctor-patient relationship, not with any symptom or medical diagnosis.
Rear Admiral Ronny Jackson served as the official White House physician under George W. Bush, Barack Obama, and Donald Trump. When his proposed promotion to Secretary of Veterans Affairs was being considered, we learned White House staff called him “the Candy Man” because on the President’s airplane, he allegedly gave out Ambiens like candy. Allegedly this was often without taking any medical history or providing any cautions. Senator John Tester described it this way: “on overseas trips, the Admiral would go down the aisleway of the airplane and say, ‘All right, who wants to go to sleep?’ and hand out the prescription drugs like they were candy and put them to sleep, and then give them the drugs to wake them back up again.” There also seem to be many hospital administrators and insurance companies that use The Candy Man’s technique for making friends and marketing their businesses.
In the CPSI study, about a third of people who said that they took sleeping pills “often” said that they never had insomnia. Remarkably, even recent studies show that most people given sleeping pill prescriptions do not have complaints or diagnoses of insomnia in their medical records. This suggests that gift-giving or doctor-marketing explains much hypnotic prescribing.
We should not blame the physicians alone. Patients like to receive gifts! They like to feel that they are taking something which might help, even if there is no scientific evidence. In fact, patients often insist that they need sleeping pills, and may become quite irate if a doctor does not want to give in to their sleeping pill requests. When I talk to physicians about sleeping pills, they tell me these stories again and again. Most physicians try to be ethical about sleeping pills, but they also realize that the patient given a sleeping pill may be satisfied and more likely to return for a renewal prescription, whereas the patient refused a sleeping pill may make complaints, write bad reviews, or look for another doctor. Doctors are fond of their patients and like to keep them.
A physician concerned about combined opioid-benzodiazepine overdoses told me in 2018 about her experience with trying to get hospital administration to cut down on medically-unjustified sleeping pill prescriptions. The hospital administration refused, saying in effect that cutting down on sleeping pills would hurt hospital marketing. “Marketing” is a polite way of describing giving addicting drugs to patients without a medical indication. The hospital doctor learned that almost one quarter of San Diego Medical Examiner cases dying of opioid overdoses had recently been prescribed zolpidem. Zolpidem prescriptions are associated with markedly elevated opioid overdose rates. Over the past three years, hundreds of state, county, city, and tribal governments have brought suits against the makers of oxycontin and other opioids for marketing that included giving out free samples of addictive drugs and misleading advertising minimizing risks. We applaud the public prosecutors for taking on the rich and well-connected prescription drug industry. Prosecutors may soon realize that sleeping pills are part of the opioid epidemic, and in some cases, supplied by the same companies that supply oxycontin and other opioids.
2.E. The problem of addiction.
All U.S.-approved prescription hypnotics are addicting (with the exception of ramelteon and the new drug Silenor). By addicting, we mean that these sleeping pills have two properties. First, when we take addicting drug such as narcotics or barbiturates, we develop tolerance so that a given dosage has less and less effect or “stops working.” People who develop tolerance are prone to increase their dosage more and more. Second, addicting drugs cause physical withdrawal symptoms when addicts try to stop. The withdrawal symptoms of hypnotics such as barbiturates and benzodiazepines are very well known.[34] Symptoms include insomnia, shakiness and tremor, nervousness and anxiety, panic, hyperactivity and increased reflexes, rapid heart rate: even epileptic seizures and death in the most severe cases. In one sense, the withdrawal syndrome with hypnotics can be worse than withdrawal from heroin, because while the heroin addict experiences withdrawal as a terrible anguish, it is rare that addicts do not survive even the most severe heroin withdrawal. Very abrupt withdrawal from overuse of sleeping pills can produce death. The risk of seizures and death is probably more severe with withdrawal of barbiturates than with benzodiazepines. Zolpidem (Ambien) seems less prone to cause withdrawal symptoms than the barbiturates or older benzodiazepines, but that does not mean that zolpidem is free from withdrawal risks. As compared to heroin, the withdrawal syndrome may be more lasting with the hypnotics, perhaps more than a month in some cases, though too little controlled experimentation has been done to have detailed information.
The addicting properties of hypnotics manifest themselves in several ways. Triazolam (Halcion) is such a short-acting drug that many people used to take bedtime doses which (for the first hour) were much stronger than the initial dose of a drug such as flurazepam or temazepam. But because triazolam disappears from blood largely with two to three hours, some people find themselves in triazolam-withdrawal before morning. As a consequence, people taking triazolam may experience increased early awakening.[35] I suspect that zaleplon (Sonata) may be similar to Halcion in this regard, since it scarcely increases total sleep time. The manufacturers have admitted that zolpidem (Ambien) and eszopiclone (Lunesta) can also cause this early awakening. Although the risk may be less with Ambien CR, it is not always eliminated.
Next, by wake-up time, the person taking zaleplon or triazolam or zolpidem will certainly be approaching withdrawal. The result, in at least some patients, may be increased tension and anxiety during the day.[36] I have seen two patients who developed daytime panic attacks for the first time while taking triazolam. After withdrawing from this sleeping pill, the panic attacks of these patients disappeared. One might expect that anxiety symptoms might develop somewhat later in the day with temazepam (Restoril) or estazolam (ProSom), because of the slower decrease in blood concentration, but shifting withdrawal later in the day might make trouble falling asleep even worse.
Almost any patient discontinuing any of the short-acting benzodiazepines might experience some sense of anxiety and some withdrawal insomnia after discontinuation. Doctors argue whether the withdrawal syndrome universally leaves patients worse than they would be without the drug, but I suspect it often does. These symptoms make it very difficult for patients to stop using these drugs once they have become habituated to them. Sometimes very long-term usage results, because the patient finds too much difficulty withdrawing.
The drug companies and many “outside” experts on company payrolls would emphasize that most people who take sleeping pills use them for less than 15 doses in a year and do not become habituated. It is good that not every patient who tries sleeping pills becomes an addict, but the long-term users take so many pills (often 365 or more per year) that most of the hypnotic prescriptions sold go to these chronic users. For example, in our CPSII data, 65% of the sleeping pills reported taken in the past month were taken by people reporting that they took at least 30 doses per month, and these patients reported taking sleeping pills for an average of five years. It gives quite a different picture of the sleeping pill industry, when we realize that they are profiting primarily from chronic users who have become habituated or physically addicted to these medicines. For the drug companies, one addict is more profitable than a dozen people who only try sleeping pills on rare occasions, such as after long airplane flights.
Studies of barbiturate addicts showed that while taking huge doses of these sleeping pills, many addicts slept very little. In some cases, after a long and unpleasant withdrawal, the abstinent addicts found themselves sleeping more than they had been while taking high barbiturate doses. It seemed that long-term usage of the barbiturates had actually decreased sleep. Whether a similar phenomenon occurs with the benzodiazepines is uncertain, but it is a possibility. Certainly, the CPSII study and similar studies show that people who use sleeping pills on average report sleeping less than people who do not use them, although that relationship does not distinguish which is cause and which effect. It appears that patients who stop chronic sleeping pill use may find that their sleep actually improves, as many cognitive-behavioral therapy trials have proven.[37] Maybe it becomes a circular process, where people take sleeping pills because of poor sleep, but sleeping pills cause poor sleep. The situation may be similar to that with alcohol, which can be a sleep-inducing drug with a very short half-life. I know of little study of how much alcoholics sleep while they are drinking, but after abstinence, abstinent alcoholics sleep very poorly, and they are unable to obtain a normal sleep duration. It appears that in the long run, chronic usage of alcohol damages the sleep system.
One advantage of some over-the-counter sleeping pills is that there is less evidence that they cause habituation and addiction.
2.F. Strange sensations of benefit.
Studies find that sleeping pill users often describe greater increases in their sleep than any increases that EEGs record. Controlled trials show that sleeping pills fool people into thinking they receive more benefit than can be medically confirmed. An example was the Dalmane-midazolam study, where the insomniacs said that the drug was helping, even when after 14 days, there was no benefit either by EEG measurement or even by patients’ own estimates of how long they had slept.
Amnesia explains why patients think sleeping pills help more than they do. Testing proves that sleeping pills erase memories from the night. In the past, many of the over-the-counter sleeping pills contained scopolamine, an anticholinergic drug which caused amnesia but has no substantial sleep-inducing effect. Presumably, scopolamine affected the memory of insomnia rather than its actuality. It just helped people forget how poorly they might be sleeping. Sleeping pills mainly make people forget how much they were awake at night.
Benzodiazepine agonists make people less aware of their awakenings or less disturbed by them, partly because the drug may produce a sense of well-being, as other addicting drugs do. Indeed, any number of studies have documented that patients like how they feel when they take sleeping pills. To give perspective, let me mention that people also like how they feel when they take heroin or excessive alcohol. A good feeling does not mean that taking a feel-good drug is wise.
Some dying people near the end of life want medications to ease their pain when they are beyond medical cures, even if it might further shorten their lives. Most people who take sleeping pills are a long way from being ready to die. Regardless of whether you agree with assisted suicide, most patients who seek sleeping pills are not ready for this assistance; indeed, they do not even have insomnia.
2.G. Disinhibition of punished behaviors and the dark side of tranquilization.
To understand why people continue taking benzodiazepine hypnotics when experiments show they improve sleep so little but impair performance, it may be helpful to discuss some side-effects of these drugs on behavior. In experiments where a laboratory rat will receive an unpleasant shock when it presses a lever, an animal given a benzodiazepine will be more likely to press the lever than an animal given placebo. Scientists say that benzodiazepines disinhibit punished behavior, which means that the animals become more likely to hurt themselves or to behave in a way in which they will be hurt. Another way of saying this is that benzodiazepines disinhibit aversive behaviors. There is a human analogy.
In humans, an action of benzodiazepines is to reduce fears of being harmed, which we may call being tranquilized. People very much like this feeling of reduced fear, and there is no doubt that many people like how they feel when taking benzodiazepines. Unfortunately, this tranquilization effect reduces a person's healthy fear of self-destructive actions. For example, as with alcohol, a person driving 80 mph down the highway approaching a curve ought to slow down for the curve. Taking a benzodiazepine might make a driver less likely to slow down. In some studies, benzodiazepines make people more likely to be physically aggressive, perhaps just as alcohol may make people ready to fight. This blunted fear of harmful behaviors or blunted anxiousness to protect oneself may be one way in which sleeping pills cause falls, auto crashes and shorten people’s lives.
There is another curious twist to this idea. When we consider that benzodiazepines increase people’s tendency to act in a self-harmful way, it is logical that taking harmful sleeping pills may be one of the harmful behaviors which benzodiazepines tend to increase.
2.H. Infection.
Working with colleagues at Scripps Clinic, we found that people who take sleeping pills such as eszopiclone, zaleplon, and zolpidem have about a 44% higher risk of developing infections such as sinusitis, pharyngitis, upper respiratory tract infections, influenza, herpes, and so forth.[38] There has been almost no recognition of this risk in the medical literature, but it is statistically extremely convincing, based on studies which the manufacturers submitted to the FDA and some of their own published controlled trials. The manufacturer of Ambien has admitted to the FDA that their own data confirm this adverse effect.
One mechanism is that zolpidem (and probably other sleeping pills) relax the stomach valve and cause gastro-esophageal regurgitation. The acid irritation may lead to infection. Incidentally, acid regurgitation may also lead to esophageal or lung cancer, which are among the cancers most greatly increased among sleeping pill users. At present, we do not know entirely why these infections occur, but it does seem that infections would be sometimes annoying, sometimes painful, and sometimes frankly dangerous.
It is not clear if ramelteon has the same risks, but there is one table in FDA data which suggests that it might. We could not find adequate information concerning the older sleeping pills. A new study from Great Britain showed that use of benzodiazepines (including popular older sleeping pills) was associated with a 50% increase in hospitalizations for pneumonia and about a 30% increase in subsequent mortality. For more scientific data about infections, see this review: [39]

Good Sleep Habits and Attitudes: Cognitive-Behavioral Therapy of Insomnia (CBT-I)
The better alternative to sleeping pills is to develop good sleep habits and good sleep attitudes. Good sleep habits and attitude are the best approach for a long-term sleep problem, and they produce surprising improvement.[40] In this chapter, we start with presenting the thinking (cognitive reasoning) behind good attitudes and then the cognitive-behavioral therapy of insomnia.
First, remember that most people do not need eight hours of sleep per night. That old idea just is not so. People with financial connections to sleeping pill manufacturers are trying to preserve the eight hours belief that never had much evidence behind it. In our studies in San Diego, the average adult was recorded asleep only between 6.0 to 6.5 hours a night. National polls give similar results. Moreover, in the Cancer Prevention Study II study of over a million Americans, people who said they slept 6.5 to 7.5 hours lived a bit longer than people who slept eight hours or more. The shorter sleepers lived longer! Even some groups who said that they slept as little as 3.5 hours lived longer than similar groups who slept eight hours or more! In a group of women over age 65 who volunteered for the Women’s Health Initiative, wrist recording indicated that they slept about an hour less than they thought they had slept. According to those recordings, volunteers who slept 5.0 to 6.5 hours had the lowest mortality.[41] If you feel you sleep five to seven hours a night and feel rested, there is no evidence that you should try to sleep any more as far as life expectancy is concerned, and that is largely true of other health measures. For example, there is more heart disease among those who sleep more than eight hours. Incidentally, in some studies controlling for other illnesses, age, and so forth, people who said that they had insomnia lived a little longer than those who did not have insomnia! Therefore, do not worry too much about insomnia!
· Some groups who said that they slept as little as 3.5 hours lived longer than groups who slept
eight hours or more.
· People who
said that they had insomnia lived a little longer than those who did not have
insomnia.
· There were MORE DEATHS related to sleeping eight hours or more than there were related to sleeping less than 6.5 hours.
Short sleep is associated with good health as well as long life. Studies show that in the range that most Americans sleep (which is six to eight hours or so), there are few discernable differences between people. This may surprise you, but people who sleep six hours seem to be at least as happy as people who sleep eight hours. Moreover, people who sleep six hours get just as much work done and are just as rich as people who sleep eight hours. There may be some tendency for people with the shortest sleep times (five or six hours) to be outgoing and energetic, whereas people with the longest sleep times (nine or 10 hours) seem to be more introverted, imaginative, or perhaps a bit depressed, and they are more likely to be unemployed. Notice the surprise! People who sleep less are often less depressed!
Indeed, hospital studies of depressed patients show something very remarkable. When depressed patients are kept awake all night (or at least for the second half of the night, e.g., after 2 a.m.), they describe feeling less depressed the following day. Being awake at night lifts a depressed mood. Moreover, after the wake therapy, taking a nap makes depressive symptoms recur. Wake therapy would be a very popular treatment for depression except for one problem: people with depression who stay up all night do get sleepy, and after they sleep soundly the next night, the low mood relapses. In my ebook Brighten Your Life, I explain how this relapse can be avoided with bright light. It is true that people who are getting depressed may have poor sleep, but it is not proven that getting more sleep helps depression. It may be quite the opposite. In fact, it has now been proven that cognitive-behavioral therapy that restricts time in bed improves the mood of patients with insomnia. Less time in bed can sometimes lessen depression.
For these reasons, depressed people usually should not struggle to get more sleep, and should certainly avoid sleeping pills, which tend to cause depression.
Many people may improve their moods by getting up a bit earlier.
There is another factor. Spending too long in bed – as you might expect – causes people trouble with falling asleep and makes them more likely to wake up in the middle of the night. Sometimes, the frustration of lying in bed awake adds to the problem, and it builds on itself, getting worse and worse. The more time the person spends in bed trying to get more sleep, the more trouble can develop in falling asleep and the more the person may awaken in the night. Surprisingly, it seems that spending too long in bed might be a major cause of sleep trouble among both elderly and depressed people. One expert remarked that the false belief that people should sleep eight hours is one of the major causes of insomnia. Fortunately, there is an easy solution.
People who are spending a lot of time in bed lying awake should spend less time in bed. This means either going to bed later or getting up earlier. Getting up by a regular time seems to be important, so trouble falling asleep should not persuade you to sleep late. The less time you spend in bed, the more you will feel sleepy the next evening and the more easily you will fall asleep. Think about it. If you spend less time in bed, you will surely tend to fall asleep more easily and sleep more soundly in the future. Moreover, the less time you spend in bed, the more you are likely to restore the habit of falling asleep quickly after going to bed, and the more you improve the habit of sleeping soundly. Some doctors recommend that at the beginning of cognitive-behavioral therapy, you should avoid spending more time in bed than you currently think you sleep. For example, if you think you only sleep 5½ hours a night, spend only 5½ hours in bed until you are sleeping all 5½ hours. Then you can try increasing time-in-bed about 15 min., e.g., to 5 hours and 45 minutes. You can gradually increase your time in bed on a weekly basis until you are no longer sleepy enough to sleep at least 85% of your time in bed. Once you are sleeping no more than 85%, that is the longest bed time that you should allow yourself.
One warning: when you have first reduced your time in bed, you will feel more sleepy. Be cautious because that new sleepiness could cause problems with driving and other tasks. The sleepiness helps you sleep more soundly, but in the day, restriction of time in bed causes discomfort and minor risk before you learn to sleep more efficiently while in bed.
Most sleep experts also recommend that whatever bedtime you allow yourself, you should not go to bed if you do not feel sleepy. Moreover, if you awaken at night and no longer feel sleepy, get out of bed, and do not go back until you are sleepy again and expect to fall asleep. Even after being up during the night, you should get out of bed by your regular awakening time, because sleeping late tends to make the problem worse. Getting out of bed when you are not sleepy makes you sleepier the next night and helps retrain good sleep habits.
Almost all of us have stayed up entirely for a night or two, so we know that nothing terrible happens to us. I have talked to many patients who say that they have slept only a few hours a night for years, and yet they are somehow afraid that losing sleep will hurt them. Probably not. Remember that if anything, people who sleep a bit less than average tend to live longer and be less depressed. If you are willing to stay out of bed and amuse yourself somewhere else when you are not sleepy, soon you will stop worrying about sleep. If you lose a whole night’s sleep or part of a night, so what? It will not be so bad, so long as you do not worry about it. When you do go to bed (because you are finally sleepy), you will have restored your confidence that you are likely to fall asleep, so the long-term problem resolves.
If you do begin to worry about how a bad night of sleep will affect you the next day, remember that it is a very poor idea to take a sleeping pill. The sleeping pill is likely to make your performance worse the next day, and very unlikely to help.
Experts also advise that you avoid worrying in bed, watching TV (especially those scary late-night movies), reading scary mysteries, and doing other upsetting things besides sleep and sex in bed. The idea is not to make a habit of being worried or alerted in bed. If you are a person who worries, select a place to worry (such as a chair in another room), and sit down to worry there. When you are tired of worrying, then go to bed.
Good sleep habits also require avoiding coffee or anything else with caffeine within six hours of bedtime. Alcohol is sometimes a cause of sleep trouble, because although alcohol may relax us at first, it leads to insomnia as soon as the blood alcohol level falls. Drinking early in the evening may cause trouble falling asleep. Drinking at bedtime may cause midsleep awakenings and early awakening.
People say that exercise helps sleep, but I think the real exercise benefit is minimal. Probably it is being outdoors in daylight (often where people exercise) which is most helpful. We have found that people who spend more time in daylight have fewer sleep problems. For more information about this, see my online ebook, Brighten Your Life.
Adopting good sleep habits and attitudes is extremely effective in solving long-term sleep problems. It is more effective than sleeping pills.[42]
If good sleep habits and good attitudes do not solve your problem, there is a good chance that you are suffering from depression. You should consult your doctor. You can read more about treatment of depression in my online ebook, Brighten Your Life. You might also consult a sleep specialist at a sleep clinic. You might have a problem with your body clock (which I describe in Brighten Your Life) or another sleep disorder which could benefit from specific specialist treatment or self-treatment. For a chronic problem, I advise against asking a doctor for sleeping pills. It is the wrong approach.
For help with insomnia by changing habits and attitudes, try a program of Cognitive-Behavioral Therapy for insomnia, abbreviated CBT-I. A good therapist might be most helpful, but if you can’t find a CBT therapist in your community, you can get much of the same benefit from pamphlets, books, or the internet. A Smart Phone App called “CBT-I Coach” has been available free from Android (Google Play) and iOS (Apple Store) download sites. It was developed by U.S. experts with support from the Veterans Administration. There are also growing number of developed commercial internet web sites which may cost less than a single therapist visit, e.g., CBTforInsomnia.com[43] and SHUTi[44], but I have no recent experience with either of them. There are other CBT-I programs in the U.S. that I know less about and also good CBT-I web sites from the United Kingdom (e.g., www.Sleepio.com) and other parts of Europe. The Veterans Administration is making available on-line an increasing number of informational materials to help people get away from sleeping pills, besides the CBT-I Coach cell-phone app.
CBT-I helps more than sleeping pills and CBT-I is much safer. An exhaustive literature study sponsored by the U.S. government Agency for Healthcare Research and Quality (AHRQ) concluded that CBT-I produced much more definite sleep improvements than sleeping pills, and the CBT-I produced far less evidence of serious bad effects.[45] The AHRQ analysis found CBT-I to be better, even though the AHRQ methods had been biased in favor of sleeping pills because: 1) AHRQ only considered subjective patient evaluations (ignoring objective sleep recordings that are known to find less drug benefit), 2) AHRQ considered only published articles (known to be biased because drug companies tend to avoid publishing poor trial outcomes), and 3) AHRQ gave much more attention to possible benefits than important risks. In reporting even “low-strength evidence” for weak sleep benefits from hypnotics, the AHRQ report failed to mention that they had not confirmed any benefit from the recently-reduced recommended-low doses of drugs such as zolpidem, eszopiclone, and suvorexant.
Interpreting the AHRQ evidence, an American College of Physicians Guideline concluded that treatment of insomnia should begin with CBT-I, not with any sleeping pill. Considering the risks, the American College of Physicians expressed doubt that sleeping pills should ever be used even for short-term treatment.[46]
There have now been dozens of randomized trial comparisons of CBT-I versus sleeping pills, showing that in the long run, CBT-I is more helpful and safer than sleeping pills.

The Benefits of Hypnotics
I have described the dark side of hypnotics and described the alternative correction of habits and attitudes, because these are the most important points about sleeping pills. I did not describe any sleeping pill benefits until this Chapter 4, because in my view, the risks of death, cancer, depression, and infection with sleeping pills, besides the behavioral impairments and accidents, are much more important than any small benefits. Besides, use of sleeping pills seems to cause insomnia, at least after withdrawal.
A laborious and somewhat misplaced effort has employed sleep researchers over the years to measure the small amount by which sleeping pills increase sleep. I will not bore you with the details. The effort is misplaced, in the sense that the prescription sleeping pills increase sleep only very little, so that the exact size of the tiny benefit is trivial.[47] In fact, at the low drug doses that the FDA recommends for safety, popular sleeping pills do not reliably increase sleep at all. The manufacturers of Ambien and Belsomra (zolpidem and suvorexant) have written the FDA that these low doses are “ineffective.” Likewise, the official information on Sonata (zaleplon) stated, “a significant difference from placebo on sleep duration was not demonstrated,” which means that zaleplon generally did not help people sleep more than a dummy pill. I think the recommended doses of Lunesta (eszopiclone) and Rozerem (ramelteon) are similarly ineffective.
In most sleep laboratory studies, sleeping pills given to insomniacs increase their self-reported sleep only 20-40 min. or even less. The EEG-laboratory recorded benefit of sleeping pills is still less than what patients report. These are only trivial increases, when we consider that many people who sleep only five hours do not complain of insomnia, whereas there are people who report sleeping nine hours or more who feel their insomnia is severe. As I have mentioned above, although 20 min. increases in sleep may be statistically significant (which means statistically reliable), they are not functionally significant, since sleeping pills usually produce no measurable improvements in daytime performance or health.
Ramelteon (Rozerem) may offer little risk (we did not have enough data in our epidemiologic study for ramelteon), but it also offers little benefit. In short-term studies, Rozerem produces a small decrease of time to objective EEG sleep of seven to 16 minutes, which is trivial. After six months, Rozerem increased total sleep by one minute compared to placebo! According to the NDA data at the FDA web sites, in many of the company studies, patients who received Rozerem did not think they were sleeping better than those receiving placebo. However, if many patients taking ramelteon do not feel they are sleeping better, why buy the stuff? I have found that many patients do not like Rozerem. We do not know about mortality, but some indications suggest that ramelteon might cause depression, infection and cancer.
“The European Committee for Medicinal Products for Human Use (CHMP) has issued a negative opinion on the use of the melatonin receptor agonist ramelteon in insomnia, due to its unfavorable risk-benefit balance.”[48] They thought melatonin itself might have a better benefits/risk ratio for treating insomnia.
I agree with the European opinion.
Whereas most sleeping pills increase sleep a few minutes for the first few nights of use, it is unclear how long the benefits last with continuous nightly usage. In our Dalmane-midazolam study, the benefits were gone in less than seven days as compared to placebo,[49] and in the triazolam-flurazepam study, the benefits were gone after three weeks as compared to placebo.[50] Unfortunately, for many years, almost all laboratory studies have used placebo baseline recordings as the control, without counterbalancing the order of placebo and hypnotic. The studies where hypnotic and placebo are given in parallel (at the same time to randomly-assigned volunteers) suggest that participation in laboratory experiments (and spontaneous recovery) led to improvements in sleep. After two to four weeks, the improvement seen in a drug-treated group as compared to baseline may be due to the time-related placebo-effect improvement rather than due to any real drug benefit. Manufacturers’ advertising often deliberately confuses this point.
Even with tiny increases in sleep that they provide for a few days, hypnotics do not improve an insomnia patient’s daytime function. More often, the pills make daytime function measurably worse. Patients often seek improved function, but they usually do not receive it. Further, although we hear colleagues mention that perhaps a patient will be healthier if the patient sleeps better, our research found that patients taking sleeping pills were more likely to develop new medical disorders than matched control patients who avoided sleeping pills. I have located no reliable evidence that any sleeping pill improves general health, but there is much evidence of serious harm to physical and mental health.

Recommendations of Experts
In 1979, a distinguished committee of our national Institute of Medicine considered the risks and benefits of hypnotics. Noting concern with the side effects and risks of sleeping pills balanced by the lack of evidence for long-term benefit, this distinguished committee recommended that hypnotics generally be limited to short-term use.[51] In 1983, a Consensus Conference held by the National Institutes of Health on the treatment of insomnia. This group recommended that sleeping pills be used mainly for up to three weeks, not longer.[52] Another consensus conference was held in 1990 to discuss problems of sleep in aging. Complaints of insomnia are much more common among people above age 60 years, and 40-50% of all sleeping pills are taken in the U.S. by people over 60. This consensus group also recommended only short-term use of sleeping pills.[53] A new committee of the Institute of Medicine concluded in 1997 that the data only supported use of Halcion for two weeks.[54] In the summer of 2005, the National Institutes of Health had a consensus conference[55] about insomnia, which emphasized how little we understand about chronic insomnia. This group of experts concluded that the evidence for behavioral therapy for chronic insomnia was better than evidence for long-term use of sleeping pills, though this group of experts failed to frankly condemn long-term sleeping pill use. In summary, there is expert consensus that the medical evidence does not support chronic use of sleeping pills.
A meta-analysis (combined analysis) of a large number of sleeping pill trials was published in the British Medical Journal, one of the most authoritative medical journals.[56] This analysis, focusing on studies of people with insomnia over 60 years of age, concluded that long-term use of sleeping pills more often does harm than good. This conclusion was reached without considering risks of mortality and cancer, which further tip the likelihood towards harm. More recently, the American Geriatrics Society recommended that older patients avoid benzodiazepines or benzodiazepine-like sleeping pills in all situations.[57] To follow this recommendation would immediately eliminate about half of the sleeping pill prescriptions in America.
As mentioned, The American College of Physicians guidelines suggested that CBT-I always be tried before sleeping pills. The Guidelines expressed doubt that sleeping pills should ever be used, even short-term.[58] Similarly, the European guideline for treatment of insomnia recommended that CBT-I be tried before hypnotics, and emphasized both the weakness of evidence for hypnotics benefits and the strong evidence for serious harm to physical and mental health.[59]
In conclusion, most experts without financial ties to the sleeping pill industry have reached the same conclusions as mine even before seeing our 2012 data about mortality and cancer. Since 2012, much more evidence about the risks of sleeping pills has accumulated. Drug companies have marketed an impression that sleeping pills are accepted by spending hundreds of millions of dollars promoting their drugs on TV and with direct marketing to doctors. Drug companies supply doctors with free lunches, trips to conferences in luxury hotels and large “speaking fees” once they have arrived to persuade doctors to listen to their propaganda. Despite all this, the opinion of the majority of experts without financial conflicts agrees with what you will read in this ebook.

Getting Off Sleeping Pills
As I have explained, because of mortality, cancer, depression, infection, and behavioral risks, I cannot recommend circumstances when anybody should take zolpidem, eszopiclone, zaleplon, temazepam, triazolam, flurazepam, estazolam, quazepam, barbiturates, or diphenhydramine as hypnotics.
The manufacturers generally claim that a person taking only the recommended dosage each night should safely be able to stop the pill immediately. Many experts feel that patients who have been taking higher doses or a modest regular dosage for a long time may need to slowly taper off the medication, reducing their dosage by a small portion every week or two. Even with slow tapering, withdrawal from sleeping pills can cause at least a few nights of insomnia, anxiety (both day and night), tremulousness, and other symptoms. People will have much less difficulty withdrawing from sleeping pills if they first begin CBT-I treatment as described in Chapter 3 above, or obtain CBT-I from a therapist or web site.
It is always recommended that a patient consult the prescribing doctor before discontinuing a prescribed sleeping pill. A doctor’s supervision is particularly important for patients withdrawing from higher doses.
For most patients, it will not be necessary to replace a sleeping pill with any other drug merely for treatment of insomnia. If related illnesses such as depression, anxiety, etc. are involved, an approved medication, CBT-I, psychotherapy, or bright light treatment for those conditions may be needed. Even people with no intrinsic depression or anxiety are likely to become anxious when withdrawing from a sleeping pill. It helps to understand that this anxiety and fear of insomnia is usually a drug withdrawal reaction which will go away in time, often within a day or two, so starting a replacement drug may not be advisable. People withdrawing from sleeping pills may become filled with the idea that they can never do without their pill, when a few days later, they do perfectly well without it.
There are some drugs which could be substituted for those sleeping pills that I have advised discontinuing because of mortality and cancer risks. I do not say that I recommend such substitution. Certainly, I would not recommend substituting in ordinary circumstances, but I recognize that physicians will encounter some patients for whom at least short-term substitution seems a good idea. I do not think that the possible substitutes have been shown to be associated with mortality. The relationship to cancer for these drugs seems to me uncertain.
The most reasonable substitute drugs might be trazodone, Silenor (doxepin 3 or 6 mg.) and melatonin, but I say this without recommending substitution. Trazodone and melatonin are not FDA-approved as hypnotics as of September, 2018.
Trazodone has been shown to be somewhat effective as a hypnotic in low doses (in higher doses, it is an effective antidepressant), but trazodone has worrisome side effects. Trazodone has recently been very popular in the United States for off-label use as a hypnotic, which seems to indicate that patients and doctors like it. I have seen good results myself, but I have also seen some bad side effects such as falls and excessive daytime sedation. Use of trazodone as a hypnotic is not FDA-approved, and little is known about trazodone’s long-term safety.
Silenor in early reports seems to be somewhat effective for maintaining sleep, though of less use for helping people fall asleep. I am not convinced that we have enough experience with use of Silenor to be sure of its safety, and I have not personally seen patients who are doing well with Silenor. It has been claimed that Silenor is lacking in significant side effects at doses of 6 mg. and below, which might be believable, since we formerly prescribed up to 300 mg. of doxepin to treat depression. Time will tell. Silenor is currently FDA-approved as a hypnotic.
Melatonin in an immediate release form sometimes has a benefit in slightly reducing the time to fall asleep, but it is less effective or ineffective in prolonging sleep later in the night, so its benefits for total sleep time are often weak or absent. Melatonin may accelerate sleep onset best when given an hour or more before bedtime. Melatonin is a night-timing drug, not a hypnotic as such. Night-active rodents have the highest melatonin blood concentrations when they are wide awake. There is evidence that melatonin has a variety of minor side effects such as headache and nightmares, and some little-studied effects on the reproductive endocrine system, but little or no evidence in humans of serious side effects. A sustained release melatonin preparation (Circadin) has been approved as a sleeping pill in Europe. Initial published reports suggest that Circadin has a favorable benefits/risks ratio. However, there seems to have been a trend to leave the less favorable studies of sustained release melatonin unpublished.[60] I confess I am skeptical of drugs whose manufacturers tend not to publish the less favorable studies, although it is a common failing of pharmaceutical manufacturers. As of this writing, sustained release melatonin is not yet FDA approved in the U.S. as a hypnotic. Our research suggested a trend for older women who secreted more natural melatonin to have higher mortality, but this trend was not statistically significant.[61] I think we need more long-term studies of melatonin safety.
A specific use for melatonin is treating people with delayed sleep phase disorder (nightowls who have trouble falling asleep and trouble getting up in the morning). There is considerable evidence that very low doses of melatonin (50-500 micrograms) may be useful for these patients. The recommended dosage is much lower than the 1-5 mg. (1000-5000 micrograms) usually sold over the counter. As I have mentioned, I agree with the European Committee for Medicinal Products for Human Use (CHMP), which thought that melatonin would have a better benefits/risks ratio than ramelteon.
Suvorexant is an approved hypnotic, but not enough is known about its interactions with other hypnotics for me to recommend its substitution for patients withdrawing from other hypnotics. Suvorexant seems to have particularly dramatic withdrawal effects.[62]

How Much Are Sleeping Pills Used in the United States?
I do not think anybody has reliable information on how much Americans take sleeping pills. Most scientific discussion has cited data from the National Prescription Audit, a survey system conducted by IMS America, Ltd. (now part of IQVIA). Their survey methods were proprietary, and I do not know in detail what they were, but they involve computerized monitoring of retail pharmacy sales. According to the Wall Street Journal, IMS Health data showed about 60 million prescriptions for hypnotics in the U.S. in 2010.[63] I believe this 60 million may have been an underestimate, but the number of hypnotic prescriptions dropped after 2012. Considering both United Nations data[64] and the newspaper reports of IMS data, I have estimated that about ten percent of U.S. adults were probably taking sleeping pills in 2010, and the percentage will probably exceed seven percent in 2018. The U.S. government seems to have no clear data about the use of sleeping pills, even though most are addicting drugs regulated by the Drug Enforcement Agency.
At some personal expense, I filed Freedom of Information requests, asking the FDA, the Drug Enforcement Administration, and Customs what the sales of hypnotics were in the United States. Only by threatening legal action was I able to get U.S. government agencies to admit that they did not have the information. I believe it. I believe that the U.S. government does not know how many sleeping pills Americans use and what percentage of Americans use them. The government agencies also have only vague information about how often misuse of hypnotics is combined with misuse of narcotics, though the combination increases the overdose risks. Considering that the hypnotics are addicting drugs and drugs of abuse, I think our government ought to pay better attention.

Why Haven’t You Heard This Opinion of Sleeping Pills From Every Expert?
The idea that sleeping pills have a dark side is nothing new, as shown by the quote from an 1893 textbook by Dr. Woods. See Chapter 5 for expert opinions and guidelines. Indeed, generations of physicians have shared my negative opinion, based on their own clinical experiences. Probably, the majority today agree. They are a silent majority, with little to be gained by making their opinions public.
The sleeping pills industry has had billions of dollars of yearly sales, and it has thought of many subtle ways of keeping its products popular. To be frank, the manufacturers of sleeping pills have often given the leaders of sleep research large monetary grants to test their products. As a young scientist, I did some of that testing myself before I saw that sleeping pills were doing more harm than good. These research colleagues are very nice people who are not the sort to bite the hand that feeds them. Some of the most prominent leaders of sleep research have been supported mainly by drug company grants and consulting fees. I still receive drug company offers, though now I refuse them. The drug companies have used many subtle free offers and not-so-subtle methods of influencing the wider group of sleep clinicians to mute their critical attitude towards sleeping pills.
For example, several years ago, manufacturers offered free chocolate cream pie at a national sleep meeting for attendees to watch a bizarre comic session in which leaders of the sleep community mocked the FDA for its efforts to regulate sleeping pills. I suppose a good deal of money was spent for those free chocolate cream pies and the advertising of that ridicule of the FDA.
For many years, the National Sleep Foundation launched a yearly publicity campaign about the dangers of insomnia, encouraging everybody to sleep eight hours. Scientific evidence to support eight hours sleep is almost nonexistent. Could this campaign have been influenced by the fact that much of the National Sleep Foundation’s money came from sleeping pill manufacturers? The public relations firm for Ambien bragged that National Sleep Foundation publicity was effective in increasing sleeping pill sales.[65] I believe more recently, the National Sleep Foundation has broadened its support to mattress and pillow manufacturers and I do not know what other groups with something to sell. The Foundation no longer details their sources on their web site.
The American Academy of Sleep Medicine received strong financial support from sleeping pill manufacturers in the past, but they do not seem to openly list that financing any more.
Unfortunately, almost nobody advertises for behavioral treatments of insomnia or for hypnotic abstinence. The advertising for bright light treatment is minuscule compared to pharmaceutical advertising.
8.A. Why haven’t you heard from the FDA?
When we reported that people who took sleeping pills died 4.6 times faster and suffered more cancer,[66] I made quite certain that the FDA had reviewed the new studies. Also, by that time, the Agency’s own internal documents showed that the FDA knew that for the majority of patients, Ambien doses were likely to be ineffective, unsafe, or both.[67] Forgive my naivety. Ignoring, at that time, 21 studies showing that people who take sleeping pills die sooner or suffer more cancer, some suggesting that sleeping pills were as dangerous as cigarettes, the FDA still claimed (in August, 2012) that sleeping pills were “safe and effective.” It may not surprise you that just a few years later, the FDA public relations person who made that “safe and effective” claim for hypnotics was employed by a sleeping pill company, no doubt well-paid. The FDA decided to require no black box warnings about mortality and cancer risks for zolpidem and similar drugs, nor did the FDA require further studies to confirm whether the mortality and cancer risks are substantial.
When the FDA took no practical steps to warn the public about sleeping pill mortality and cancer risks, and gave only minimalizing mention of proven depression and infection risks, I formally petitioned the FDA in October 2015 to require more warnings and more research to clarify how much sickness and early death were caused by sleeping pills. Although the law required (as the FDA admitted) that the Agency respond to my petition within six months, the FDA gave no substantial reply, responding mainly that the problem was too complex for them. Also, evidently fearing that my concerns would be proven well founded, neither the FDA nor the manufacturers dared to initiate the large clinical trials needed to more accurately determine the magnitude of the unnecessary mortality and disability that sleeping pills cause.
Perhaps we should not be surprised when the FDA fails to protect the public. A 2009 report of the Congressional Government Accountability Office questioned the FDA’s ability to protect Americans from unsafe medical products.[68] A 2012 National Institute of Medicine report found that the FDA’s current oversight was not adequately assuring the safety of marketed drugs.[69]
The FDA Amendments Act of 2007 gave the FDA authority to require additional safety studies on marketed drugs when needed, but these were not ordered for sleeping pills. The Act provided authority to require risk evaluation and mitigation strategies, but the FDA disclosed no such evaluation and mitigation strategy to deal with cancer and mortality risks of sleeping pills. To give another example, in a September, 2012 lawsuit and press release, Public Citizen alleged that the FDA was acting unlawfully in failing to protect the public from an Alzheimer’s Disease drug, because it had “chosen to support the profit interests of a large pharmaceutical company.”[70]
A rapidly-increasing body of evidence has demonstrated serious risks of hypnotic drugs (sleeping pills). These were documented in more than 50 new publications since 2015.[71] To better define the causal magnitude of hypnotic harms, in 2015 I had petitioned the U.S. Food and Drug Administration (FDA) to require large randomized placebo-controlled safety trials. In December 2018, when the FDA finally got around to replying to my petition – more than two years past their mandatory deadline – I was appalled that the Agency’s response ignored all 50 new publications and declared no need for further research. [72]
How did the FDA’s long-delayed response manage to paper over the increasingly obvious risks of sleeping pills? The Agency offered the outrageous claim that hypnotic mortality risks were not important so long as they did not consistently double a patient’s risk of dying: “While a few studies reported statistically significant hazard ratios of 2.0 or higher, most found either a significant hazard ratio below 2.0 or no significant association between hypnotics and all-cause mortality.”[73] This was a statistical falsification, since as of November, 2018, there were 11 studies reporting significant hazard ratios of 2.0 or higher,[74] which is more than a few. Note that the FDA did not mention that all of the 22 adequate-sized studies (with at least 14,000 participants) had found statistically significant mortality hazards for hypnotics. If smaller studies were included, 35 of 46 had found statistically significant hazards.
Does the FDA follow a policy of approving drugs that kill patients if the hazard ratio is less than 2.0? The FDA reported no meta-analyses of their own and ignored the published independent meta-analysis confirming significant mortality hazards associated with hypnotics,[75] for example, a mortality hazard ratio of 1.73 associated with the Z-drug hypnotics. The FDA was even defending FDA medical approval of barbiturate sleeping pills that nobody recommends any more except for death row.
The FDA claimed that studies of cancer risk did not support an association of hypnotics with increased cancer risk, failing to report any meta-analyses of their own and ignoring two published meta-analyses that inferred significant cancer risks. There were randomized controlled trials data from FDA files showing an excess of cancers among patients randomized to hypnotics.[76] The FDA claimed “lack of clear biological mechanisms,” ignoring evidence already revealed in FDA files that some hypnotics are clastogenic (cause mutations of chromosomes).[77] The FDA did not employ their animal testing facility to clarify hypnotic carcinogenicity in rodents, though the Agency could have supplemented the manufacturers’ inadequate studies that had already reported evidence of carcinogenicity in animals.
The FDA further ignored summaries of randomized clinical trials taken largely from FDA files which proved that hypnotics caused infections and depressions.[78] Likewise, the FDA presented no meta-analyses of their own unpublished data regarding infection and depression risks.
The 2018 FDA response endorsing 10 types of hypnotics was inconsistent with recently-issued opinions disapproving most use of hypnotics from the American Geriatrics Society, the American College of Physicians, the American Academy of Sleep Medicine, and the European Sleep Society, all of which the Agency response disregarded.[79] Why did the FDA pay no heed to these most recent distinguished expert opinions? It appears that the FDA literature review might have actually been written in 2015 without then generating a response to my petition, and that since then, the FDA has elected to ignore new scientific evidence and major new professional recommendations.
Did the FDA unveil its response to my petition on December 3, 2018 only because the November 2018 election selected a Congress that might look into FDA inaction regarding how hypnotics augment the suicide epidemic?
In the following chapter (Chapter 9), I take a satirical swipe at the Food and Drug Administration’s inaction, wondering if the FDA has in mind a secret means of reducing climate change by trimming the American population.
8.B. What if your family was injured?
If somebody in your family died or developed cancer after taking sleeping pills, you may have an entitlement to reimbursement for injury. Equally important, injured families could spread warnings. When the medical community was no match for the cigarette companies, lawyers and law suits helped disclose cigarette risks. Many state and local governments are now suing opioid manufacturers and providers for pushing opioid addicting drugs that are killing so many thousands. A substantial percentage of patients dying from opioids were at the same time receiving addicting sleeping pills and similar benzodiazepine agonists, for which similar litigation is needed.
We now need litigation for failures to disclose sleeping pill risks. If your family member died while regularly taking sleeping pills, and that risk was not disclosed, consult your attorney. If your family member developed a cancer while taking sleeping pills, especially esophageal or lung cancers or lymphoma, and that risk was not disclosed, consult your attorney. In Wyeth v. Levine[80], the U.S. Supreme Court ruled that drug manufacturers are liable if failing to warn patients, even when the FDA has not required a warning in the labeling. If the drug companies, the many doctors taking gifts and money from drug companies, the FDA, and the insurance systems will not alert the public to the risks of sleeping pills, injured families might have to give the warnings through legal assistance. You may contribute to better warnings if injured patient families and whistle blowers file enough lawsuits, recovering damages like the $4.85 billion which one company paid to settle Vioxx claims.[81] You might help save hundreds of thousands of lives.

FDA Hypnotics Policy Reduces Global Warming:
A Satirical Look at the Bright Side
One wonders why the FDA would endorse hypnotic prescribing that effectively puts a troubled population segment on hospice care and enables assisted suicide. Is it possible that within the FDA is a “deep state” cabal of environmental zealots who applaud the bright side of hypnotics as a countermeasure to global warming? This possibility deserves further explanation.
Recent studies indicate that hypnotics might take as much as five years from the life span of the average hypnotic drug taker. This increased mortality has been mainly among older retired people, who should always avoid sleeping pills, according to the American Geriatrics Society.[82] By endorsing addicting hypnotics, the FDA bumps off old folks and reduces the population. In reducing the U.S. population, hypnotics would reduce those human-made pollutants that cause global warming. I concede an FDA cabal’s actions to reduce the population might have a bright side!
Hypnotics are associated with increased lung and esophageal cancer, perhaps multiplying those risks several times.[83] Apart from shortening people’s lives, those cancers might discourage patients from venturing out and driving around. The cancers could reduce auto exhaust, air pollution, and global warming. The bright side of the FDA approval of hypnotics is that increasing people’s cancer could protect our environment.
Controlled clinical trials proved that hypnotics doubled occurrences of depressed moods and added about 44% to new infections. Several studies suggested that hypnotics increased severe illnesses such as suicide attempts and pneumonia.[84] The FDA’s bright side is that those illnesses often keep people in the hospital or at home, also reducing air pollution from driving. Other risks associated with hypnotics such as falls causing broken hips, more automobile crashes, and accidents of other kinds keep injured people at home.
Hypnotic hangover causes average patients more sleepiness the next day. Generally, hypnotics make people feel more fatigued and less alert. The FDA warns against driving after taking hypnotics. Tired and sleepy people would be less likely to zoom around spewing automobile exhaust, starting fires, chopping down trees, scattering trash around the countryside, and generally damaging our environment. Perhaps this is why the FDA says sleeping pills are “effective” but then tells sleeping pill users not to drive. I have never found a study proving that people with insomnia accomplish more if they take a hypnotic drug. To look at the bright side, addicting people to hypnotics and dragging down their activity would reduce global warming.
It is hard to prove that failing to warn about hypnotics is a secret plot hidden deep within the FDA, seeking to reduce global warming. There could be a more subtle political angle. Those states that tended to have higher overdose death rates had higher 2016 percentages voting for the winning presidential candidate, who scoffed at global warming. The correlation was r=0.38. In other words, hypnotic drugs permanently “put to sleep” those who oppose environmental protection and who refuse to recognize the dangers of global warming! The FDA’s bright side is that sleeping pills tend to rid us of those who vote against saving our planet. FDA zealots might have considered suppression of anti-environment voters the most important hypnotic drug benefit!
Press reports have claimed that a White House physician called “The Candy Man” has given Ambien to multiple White House staff including the President. Allegedly, the Candy man often recorded no medical rationale in medical records, much less the relevant medical history and physical exam. An Associated Press story alleged that the President’s muddle-headed “Covfefe” tweet was attributable to Ambien.[85] Nevertheless, I found no evidence that the Candy Man was colluding with an FDA plot to prevent global warming by impairing White House staff or by assassinating the President.
As the Monty Python song says, always look on the bright side of life.

Needs For Hypnotics Research
For those drugs I listed as Sleeping Pills Associated with Significant Mortality Risk (in Chapter 1), evidence shows those pills are too risky ever to be used (except for end-of-life and hospice care). Unfortunately, the magnitudes of the risks have not been measured with sufficient accuracy. Even less is known about drugs currently being used as sleeping pills for which too little was known to list the risk. More research is needed to learn if there are any of the sleeping pills that are safe and effective enough to be used.
For potential substitute drugs such as trazodone, doxepin, suvorexant and melatonin, we do not have electronic records studies equivalent to those for the drugs I listed as having significant mortality risk, nor for the still-newer drugs in the development pipeline. Perhaps electronic records studies of the current substitute drugs will eventually reflect on any possible association with mortality and cancer. Nevertheless, for any sleeping pill kept in use or for any newer drug, we ought to have and should demand long-term controlled trials of sufficient size to confirm if the drugs do or do not cause excess mortality, cancer, depression, infection, and other serious harms.
There must be quite a few billionaires who have seen family members develop cancer or die after taking sleeping pills. One would think that charitable donors would want better information about when sleeping pills are safe and whether any sleeping pills are worth the risks. A few tens of millions of dollars donated to universities or private foundations could advance medical research about sleeping pill safety a long way.
As mentioned, I petitioned the FDA to require long-term risk studies under The FDA Amendments Act of 2007, but the FDA has effectively refused by delaying any action beyond the reasonable deadline I requested for completion of such urgent studies. If we want such studies, somebody else will have to pay for them. Meanwhile, it is time to stop using those medicines without waiting for studies that the FDA and manufacturers obviously have no intention of performing. There is plenty of evidence already now that those medications are too risky.
Lack of government curiosity about sleeping pill prescribing is exceptional. As mentioned above, government agencies denied that they had data on overall U.S. consumption of sleeping pills. The U.S. government has certainly been more careful in studying other addicting drugs.
Given their health impact, the National Institutes of Health (NIH) also have responsibility to clarify the risks of sleeping pills (see Chapter 9). NIH has made no effort. With planned budget cuts, one cannot anticipate that NIH sleeping pill research will expand. The medical insurance companies could determine from their own medical databases whether hypnotic users are developing more cancers or dying sooner. Medical insurance companies and Medicare-Medicaid programs should examine why they are paying for sleeping pills which are increasing medical costs.[86] Fortunately, the Veterans Administration health system is slowly taking increasing efforts to reduce use of sleeping pills.
In fairness, let me mention that the same heavy prescribing of hypnotics existed in Communist countries before the dissolution of the Soviet Union, and there was a similar lack of studies behind the Iron Curtain. One should not ascribe the scientific neglect of sleeping pills entirely to the profit motive. There are just too many people all over the world who haven’t enough sense to be cautious of a Candy Man.

About This eBook
I wrote these little books – both this title and Brighten Your Life – and put the books on the web, so that people in need could learn about the dangers of sleeping pills and about alternative treatments. Much of the two books is written in the same tone and language with which I explain about sleeping pills to my patients. I offer opinions and guidance even where the scientific proof is incomplete. People want a doctor’s best opinion, even when we are not certain of everything.
This is not intended to be a scientific article, but it may be useful to physicians who want to learn more about hypnotic drugs. For physicians and others who want more scientific facts, I have included many scientific references without attempting to document every opinion. This is my advice, so not every doctor will agree with everything. You can find many of the articles at a medical library or by searching the web through PubMed[87], the database provided by the U.S. National Library of Medicine. A more extensive set of links is available at my medical review.[88]

About Dr. Kripke and Disclosures of Financial Interest
Daniel F. Kripke, M.D. is a licensed physician certified by the American Board of Psychiatry and Neurology and an Emeritus Professor of Psychiatry at the University of California, San Diego. For many years, he has done research with the Scripps Clinic Viterbi Family Sleep Center. Dr. Kripke was elected a Fellow of the American Psychiatric Association. Dr. Kripke has co-authored hundreds of medical articles and has given invited lectures in 18 countries. The American College of Physicians and the European Guidelines on Diagnosis and Treatment of Insomnia have been generous in citing Dr. Kripke’s research in their new insomnia treatment guidelines.[89] In 1973, Dr. Kripke established one of the first sleep clinics in the United States. He has been treating patients with sleep disorders and doing research on sleep ever since.
Please do not contact Dr. Kripke for personal advice. Dr. Kripke is no longer seeing patients, and the California Medical Board thinks it is unethical for a physician to give personal advice to a patient he has not personally examined. You could make an appointment for a personal consultation with physicians at the Scripps Clinic Viterbi Family Sleep Center[90] or look for other sleep physicians at numerous web sites such as those sponsored by the American Academy of Sleep Medicine (AASM).[91]
Acknowledgment: I have been fortunate that my research has been supported for over 40 years mainly by the U.S. National Institutes of Health (NIH) and the Department of Veterans Affairs. The Department of Psychiatry, the Sam and Rose Stein Institute for Research on Aging, and the Center for Chronobiology of the University of California, San Diego have also supported my research, as has the Weingart Foundation and the American Cancer Society. Some years ago my laboratory received research grants from agencies of the U.S. Army and the U.S. Navy, and from 1966-1968, I was a US Air Force sleep researcher. Recently, the Scripps Clinic Viterbi Family Sleep Center has been supporting some of my research, particularly the most recent study of mortality associated with hypnotics, assisted by the help of generous gifts from private donors. Ambulatory Monitoring, Inc. (the manufacturer of the Actillume and other wrist actigraphs) and Minimitter-Respironics (makers of actigraphs); also, The Sunbox Company, Apollo Health (now part of Phillips), and Enviro-Med (which make bright light boxes) have supported our research, partly through joint research projects funded by the National Institutes of Health. In the 1970’s and 1980’s, I did sleeping pill studies with Hoffmann-La Roche and Upjohn and once consulted with Schering, but for years I have avoided accepting any fee from pharmaceutical manufacturers, so that I would be free to report this information. I also stopped accepting fees from tort lawyers or class-action attorneys. It is important that readers understand for whom an author works. Being supported largely by public funds, I have felt responsible for explaining the research results in the public interest. I appreciate this opportunity.
Endnotes for
The Dark Side of Sleeping Pills
1. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
2. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality
or cancer: a matched cohort study. BMJ Open. 2012;2:e000850![]()
![]() . [return]
. [return]
3. Kripke, DF Is suvorexant a better choice than alternative hypnotics? F1000Res. 4, 456. 2015.![]()
![]() . [return]
. [return]
4. Administered by the U.S. Food & Drug Administration, Center for Drug Evaluation and Research, Drugs@FDA “allows you to search for official information about FDA approved brand name and generic drugs and therapeutic
biological products.” www.accessdata.fda.gov/scripts/cder/drugsatfda/![]()
![]() . [return]
. [return]
5. US Food & Drug Administration new drug application (NDA) 19-908 (Ambien) memos and exclusivity summary, previously downloaded at FDA website.[return]
6. Sanofi-Aventis U.S. LLC, Prescribing Information, Ambien CR (zolpidem
tartrate extended-release) tablets - CIV, October 2010, Publication No. ACR-WFPLR-WPLR-OCT10, www.accessdata.fda.gov/drugsatfda_docs/label/2010/021774s010lbl.pdf ![]()
![]() . [return]
. [return]
7. Administered by the U.S. Food & Drug Administration, Center for Drug Evaluation and Research, Drugs@FDA “allows you to search for official information about FDA approved brand name and generic drugs and therapeutic
biological products.” www.accessdata.fda.gov/scripts/cder/drugsatfda/![]()
![]() . [return]
. [return]
8. Kripke, DF. Possibility that certain hypnotics might cause
cancer in skin. J. Sleep Res. (2008) 17![]() , 245-250. [return]
, 245-250. [return]
9. Kao, C. H., Sun, L. M., Liang, J. A., Chang, S. N., Sung, F. C., and Muo, C. H. Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clinic Proceedings![]()
![]() 87(5), 430-436. 2012. [return]
87(5), 430-436. 2012. [return]
10. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
11. Institute of Medicine. Sleeping Pills, Insomnia, and Medical Practice. National Academy of Sciences, Washington, D.C., 1979. [return]
12. Kripke, D. F., Greater incidence of depression with hypnotic use than with placebo, BMC Psychiatry 7:42.![]()
![]() 2007. [return]
2007. [return]
13. Woods, JH et al. Benzodiazepines: Use, abuse, and consequences. Pharmacological Reviews. 1992;44:151-347. PDF available at ResearchGate![]()
![]() . [return]
. [return]
14. Tinetti, ME et al. Risk factors for falls among elderly persons living in the community. N.Engl.J.Med. 1988;319(26):1701-1707. [return]
15. FDA Medical Review of Lunesta, page 2, Center for Drug Evaluation
and Research Approval Package for Application No. 21-476, available as a PDF document
at the FDA website, www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021476_Lunesta_medr.PDF![]()
![]() . [return]
. [return]
16. Hemmelgarn, B et al. Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA. 1997;278:27-31.; Betts, TA et al. Effect of two hypnotic drugs on actual driving performance next morning. Br.Med.J. 1982;25 Sept:285-852. [return]
17. Liddicoat, Laura J. and Harding, Patrick. Ambien®: Drives Like
a Dream? Case Studies of Zolpidem-Impaired Drivers in Wisconsin, presentation
to the 58th annual meeting of the American Academy of Forensic Sciences, Washington
State Convention and Trade Center, Seattle, Washington, February 23, 2006. Powerpoint
slides from the presentation are available at the New York Times website
at www.nytimes.com/packages/other/business/Ambien.2-23-061.ppt![]()
![]() . [return]
. [return]
18. Kripke, DF et al. Prevalence of sleep disordered breathing in ages 40-64 years: A population-based survey. Sleep. 1997;20:65-76.; Ancoli-Israel, S et al. Sleep disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486-495. [return]
19. Committee on Halcion, Institute of Medicine. Halcion: An Independent Assessment of Safety and Efficacy
Data. National Academy of Sciences, Washington, D.C.,
1997. PDF available for download from the National Academies Press website![]()
![]() . [return]
. [return]
20. Johnson, LC et al. Sedative-hypnotics and human performance. Psychopharmacology (Berlin). 1982;76:101-113. [return]
21. Administered by the U.S. Food & Drug Administration, Center
for Drug Evaluation and Research, Drugs@FDA “allows you to search for
official information about FDA approved brand name and generic drugs and therapeutic
biological products.” www.accessdata.fda.gov/scripts/cder/drugsatfda/![]()
![]() . [return]
. [return]
22. Judd, LL et al. Cognitive performance and mood in patients with chronic insomnia during 14-day use of flurazepam and midazolam. J.Clin.Psychopharmacol. 1990;10:56S-67S. [return]
23. Johnson, LC et al. Sedative-hypnotics and human performance. Psychopharmacology (Berlin). 1982;76:101-113. [return]
24. Kripke, DF. Chronic hypnotic use: Deadly risks, doubtful benefit. Sleep Medicine Reviews. 2000;4:5-20. [return]
25. Roth, T et al. Characteristics of chronic insomniacs examined in a multicenter 14-day study of flurazepam and midazolam. J.Clin.Psychopharmacol. 1990;10:24S-27S. [return]
26. Kripke, DF et al. Sleep evaluation in chronic insomniacs during 14-day use of flurazepam and midazolam. J.Clin.Psychopharmacol. 1990;10(Supplement 4):32S-43S. [return]
27. Mitler, MM et al. Comparative hypnotic effects of flurazepam, triazolam, and placebo: a long-term simultaneous nighttime and daytime study. J.Clin.Psychopharmacol. 1984;4:2-15. [return]
28. Walsh, JK et al. Intermittent use of zolpidem for the treatment of primary insomnia. Sleep. 2000;23:A86. [return]
29. Beaulieu-Bonneau, S., Ivers, H., Guay, B., and Morin, C. M. Long-Term Maintenance of Therapeutic Gains Associated With Cognitive-Behavioral Therapy for Insomnia Delivered Alone or Combined With Zolpidem. Sleep 40(3). 3-1-2017 https://doi.org/10.1093/sleep/zsx002![]()
![]() .[return]
.[return]
30. Liddicoat, Laura J. and Harding, Patrick. Ambien®: Drives Like
a Dream? Case Studies of Zolpidem-Impaired Drivers in Wisconsin, presentation
to the 58th annual meeting of the American Academy of Forensic Sciences, Washington
State Convention and Trade Center, Seattle, Washington, February 23, 2006. Powerpoint
slides from the presentation are available at the New York Times website
at www.nytimes.com/packages/other/business/Ambien.2-23-061.ppt![]()
![]() .[return]
.[return]
31. Drover, D et al. Pharmacokinetics, pharmocodynamics, and relative pharmacokinetic/pharmacodynamic profiles of zaleplon and zolpidem. Clin.Ther. 2000;22:1443-1461 and Lunesta Prescribing Information. [return]
32. Committee on Halcion, Institute of Medicine. Halcion: An Independent Assessment of Safety and Efficacy
Data. National Academy of Sciences, Washington, D.C.,
1997. PDF available for download from the National Academies Press website![]()
![]() . [return]
. [return]
33. Perry, SW et al. Rationale for the use of hypnotic agents in a general hospital. Ann.Intern.Med. 1984;100:441-446. [return]
34. Lader, MH. Limitations on the use of benzodiazepines in anxiety and insomnia: are they justified? European Neuropsychopharmacology. 1999;9:S399-S405. [return]
35. Kales, A et al. Early morning insomnia with rapidly eliminated benzodiazepines. Science. 1983;220:95-7. [return]
36. Committee on Halcion, Institute of Medicine. Halcion: An Independent Assessment of Safety and Efficacy
Data. National Academy of Sciences, Washington, D.C.,
1997. PDF available for download from the National Academies Press website![]()
![]() . [return]
. [return]
37. Beaulieu-Bonneau, S., Ivers, H., Guay, B., and Morin, C. M. Long-Term Maintenance of Therapeutic Gains Associated With Cognitive-Behavioral Therapy for Insomnia Delivered Alone or Combined With Zolpidem. Sleep 40(3). 3-1-2017 Sleep.
2000;23:A86.” doi.org/10.1093/sleep/zsx002![]()
![]() .[return]
.[return]
38. Joya, F.L., Kripke, D.F., Loving, R.T., Dawson, A., and Kline,
L.E. Meta-Analyses of Hypnotics and Infections: Eszopiclone, Ramelteon, Zaleplon,
and Zolpidem. J.Clin.Sleep
Med. 5(4)![]()
![]() ,
377-383. 2009. [return]
,
377-383. 2009. [return]
39. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
40. Morin, CM. Insomnia: Psychological Assessment and Management. New York, Guilford, 1993.; Morin, CM et al. Nonpharmacologic treatment of chronic insomnia. Sleep. 1999;22:1134-1156.; Edinger, JD et al. Cognitive behavioral therapy for treatment of chronic primary insomnia. JAMA. 2001;285:1856-1864.![]()
![]() . [return]
. [return]
41. Kripke, D. F., Langer, R. D., Elliott, J. A., Klauber, M. R., and
Rex, K. M., Mortality related to actigraphic long and short sleep, Sleep
Med. 12(1)![]()
![]() , 28-33. 2011. [return]
, 28-33. 2011. [return]
42. Morin, CM et al. Behavioral and pharmacological therapies for late-life insomnia. A randomized controlled
trial. JAMA. 1999;281:991-999.![]()
![]() . [return]
. [return]
43. A description of the CBTforInsomnia.com 5-week “Conquering
Insomnia” program can be found at www.cbtforinsomnia.com![]()
![]() . [return]
. [return]
44. According to its website, SHUTi (Sleep Healthy Using The Internet) “was created to help you overcome sleep problems and insomnia symptoms using techniques and strategies modeled after Cognitive Behavioral Therapy.” www.myshuti.com![]()
![]() [return]
[return]
45. Brasure M, MacDonald R, Fuchs E, et al. Management of Insomnia Disorder [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015 Dec. (Comparative Effectiveness Reviews, No. 159.) https://www.ncbi.nlm.nih.gov/books/NBK343503/![]()
![]() [return]
[return]
46. Wilt, T. J., MacDonald, R., Brasure, M. , Olson, C. M., Carlyle, M., Fuchs, E., Khawaja, I. S., Diem, S., Koffel, E., Ouellette, J., Butler, M., and Kane, R. L., Pharmacologic Treatment of Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern.Med 165(2), 103-112. 5-3-2016.![]()
![]() [return]
[return]
47. Buscemi, Nina et al. The Efficacy and Safety of Drug Treatments
for Chronic Insomnia in Adults: A Meta-analysis of RCTs. J.Gen. Intern Med.
2007; 22(9)![]()
![]() : 1335-1350. [return]
: 1335-1350. [return]
48. Ramelteon: application withdrawn. Ramelteon in insomnia: withdrawal of marketing application in patients’ best interests. Prescrire Int. 2009 Jun;18(101):114. [return]
49. Kripke, DF, Hauri, P, Ancoli-Israel, S, and Roth, T. Sleep evaluation in chronic insomniacs during 14-day use of flurazepam and midazolam. J.Clin.Psychopharmacol. 10(Supplement 4):32S-43S. 1990. [return]
50. Mitler, MM et al. Comparative hypnotic effects of flurazepam, triazolam, and placebo: a long-term simultaneous nighttime and daytime study. J.Clin.Psychopharmacol. 1984;4:2-15. [return]
51. Institute of Medicine. Sleeping Pills, Insomnia, and Medical Practice. National Academy of Sciences, Washington,
D.C., 1979. Full text available at the National Academies Press website![]()
![]() .[return]
.[return]
52. Consensus Conference. Drugs and insomnia. The use of medications to promote sleep. JAMA. 1984;251(18):2410-2414. [return]
53. Monjan, A. A. Sleep disorders of older people: report of a consensus conference. Hospital and Community Psychiatry 41(7), 743-744. 1990. [return]
54. Committee on Halcion, Institute of Medicine. Halcion: An Independent Assessment of Safety and Efficacy
Data. National Academy of Sciences, Washington, D.C.,
1997. PDF available for download from the National Academies Press website![]()
![]() [return]
[return]
55. NIH State-of-the-Science Conference Statement on Manifestations
and Management of Chronic Insomnia in Adults, NIH Consensus and State-of-the-Science
Statements Volume 22, Number 2, June 13-15, 2005. NIH Office of the Director.
Available as a PDF document at the National Institutes of Health’s website, consensus.nih.gov/2005/insomniastatement.pdf![]()
![]() . [return]
. [return]
56. Glass, J, Lanctot, KL, Herrmann, N, Sproule, BA, Busto, EU. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ 2005;331:1169![]()
![]() . [return]
. [return]
57. Fick, D. M. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society 63(11), 2227-2246. 2015. ![]()
![]() . [return]
. [return]
58. Wilt, T. J., MacDonald, R., Brasure, M. , Olson, C. M., Carlyle, M., Fuchs, E., Khawaja, I. S., Diem, S., Koffel, E., Ouellette, J., Butler, M., and Kane, R. L., Pharmacologic Treatment of Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern.Med 165(2), 103-112. 5-3-2016.![]()
![]() [return]
[return]
59. Riemann, D., Baglioni, C., Bassetti, C., Bjorvatn, B., Dolenc, Groselj L., Ellis, J. G., Espie, C. A., Garcia-Borreguero, D., Gjerstad, M., Goncalves, M., Hertenstein, E., Jansson-Frojmark, M., Jennum, P. J., Leger, D., Nissen, C., Parrino, L., Paunio, T., Pevernagie, D., Verbraecken, J., Weess, H. G., Wichniak, A., Zavalko, I., Arnardottir, E. S., Deleanu, O. C., Strazisar, B., Zoetmulder, M., and Spiegelhalder, K. European guideline for the diagnosis and treatment of insomnia. Journal of Sleep Research 26(6), 675-700. 2017. ![]()
![]() . [return]
. [return]
60. Mattila, T, et al. Insomnia medication: do published studies reflect the complete picture of efficacy and safety? Eur Neuropsychopharmacol. 2011 Jul; 21(7):500-7. [return]
61. Kripke, DF, et al. Mortality Related to Actigraphic Long and Short Sleep. Sleep Med. 2011 January; 12(1)![]()
![]() : 28-33. [return]
: 28-33. [return]
62. Kripke, DF Is suvorexant a better choice than alternative hypnotics? F1000Res. 4, 456. 2015.![]()
![]() . [return]
. [return]
63. Petersen, A. Dawn of a new sleep drug? Wall Street Journal,
D1-D4. 7-19-2011. New York, Dow Jones. Available on the newspaper’s website at
online.wsj.com/article/SB10001424052702304567604576454102061138630.html![]()
![]() . [return]
. [return]
64. International Narcotics Control Board. Psychotropic Substances:
Statistics for 2009. (E/INCB/2010/3), 1-388![]()
![]() . 2011. New York, United Nations. United Nations Publication E/INCB/2010/3. [return]
. 2011. New York, United Nations. United Nations Publication E/INCB/2010/3. [return]
65. Kripke, DF. Chronic hypnotic use: Deadly risks, doubtful benefit. Sleep Medicine Reviews.2000;4:5-20. [return]
66. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2:e000850![]()
![]() . [return]
. [return]
67. Farkas, R. Center for Drug Evaluation and Research Approval Package for: Application Number: 019908Orig1s032s034, 021774Orig1s013s015. 2013. Silver Spring, MD, FDA. [return]
68. United States. Comptroller General. General Accounting Office. FDA Faces Challenges Meeting Its Growing Medical Product Responsibilities and Should Develop Complete Estimates of Its Resource Needs, GAO-09-581, Jun 19, 2009, available as a free PDF document at the GAO website, www.gao.gov/new.items/d09581.pdf![]()
![]() . [return]
. [return]
69. IOM (Institute of Medicine). 2012. Ethical and Scientific Issues in Studying the Safety of Approved Drugs. Washington, DC: The National Academies Press. Available for download as a free PDF from the National Academies Press at dx.doi.org/10.17226/13219![]()
![]() . [return]
. [return]
70. Bradbery, Angela. Public Citizen Sues FDA for Failing to Act on Request to Ban Dangerous Dose of Alzheimer’s Drug Aricept. Public Citizen, Inc. September 5, 2012. Text of press release available online at www.citizen.org![]()
![]() . In November 2012, the FDA decided it had not made a mistake in approving the drug and rejected Public Citizen’s petition. Wolfe, Sydney. FDA Rejects Petition to Ban Aricept 23: Did Drug Companies, FDA Collude in Approving Dangerous Alzheimer’s Drug? Public Citizen, Inc. November 6, 2012. Statement of Dr. Wolfe available online at www.citizen.org
. In November 2012, the FDA decided it had not made a mistake in approving the drug and rejected Public Citizen’s petition. Wolfe, Sydney. FDA Rejects Petition to Ban Aricept 23: Did Drug Companies, FDA Collude in Approving Dangerous Alzheimer’s Drug? Public Citizen, Inc. November 6, 2012. Statement of Dr. Wolfe available online at www.citizen.org![]()
![]() . [return]
. [return]
71. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
72. Kripke DF. Petitioner requests the Commissioner of Food and Drugs require that manufacturers of each of these drugs (zolpidem, temazepam, eszopiclone, zaleplon, triazolam, flurazepam, and quazepam, in all brands and forms prescribed to treat insomnia or patient reported sleep disorders) conduct comprehensive post-market randomized placebo-controlled trials quantifying risks and benefits to patients. Accessed December 3, 2018. Silver Spring, MD, USA, U.S. Food & Drug Administration, Center for Drug Evaluation and Research, at www.regulations.gov.![]()
![]() . [return]
. [return]
73. Page 9 of December 3, 2018 Letter from FDA CDER to Daniel F. Kripke, Primary Document, Kripke DF. Petitioner requests the Commissioner of Food and Drugs require that manufacturers of each of these drugs (zolpidem, temazepam, eszopiclone, zaleplon, triazolam, flurazepam, and quazepam, in all brands and forms prescribed to treat insomnia or patient reported sleep disorders) conduct comprehensive post-market randomized placebo-controlled trials quantifying risks and benefits to patients. Accessed December 3, 2018. Silver Spring, MD, USA, U.S. Food & Drug Administration, Center for Drug Evaluation and Research, at www.regulations.gov.![]()
![]() . [return]
. [return]
74. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
75. Parsaik AK, Mascarenhas SS, Khosh-Chashm D et al. Mortality associated with anxiolytic and hypnotic drugs-A systematic review and meta-analysis. Aust N Z J Psychiatry 2016;50(6):520-533.![]()
![]() . [return]
. [return]
76. Zhang T, Yang X, Zhou J et al. Benzodiazepine drug use and cancer risk: a dose-response meta analysis of prospective cohort studies. Oncotarget 2017;8(60):102381-102391![]()
![]() ; Kim DH, Kim HB, Kim YH, Kim JY. Use of Hypnotics and Risk of Cancer: A Meta-Analysis of Observational Studies. Korean J Fam Med 2018;39(4):211-218
; Kim DH, Kim HB, Kim YH, Kim JY. Use of Hypnotics and Risk of Cancer: A Meta-Analysis of Observational Studies. Korean J Fam Med 2018;39(4):211-218![]()
![]() ; Kripke DF. Possibility that certain hypnotics might cause cancer in skin. J Sleep Res 2008;17(3):245-250
; Kripke DF. Possibility that certain hypnotics might cause cancer in skin. J Sleep Res 2008;17(3):245-250![]()
![]() . [return]
. [return]
77. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
78. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
79. Fick DM. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015;63(11):2227-2246; Wilt, T. J., MacDonald, R., Brasure, M. , Olson, C. M., Carlyle, M., Fuchs, E., Khawaja, I. S., Diem, S., Koffel, E., Ouellette, J., Butler, M., and Kane, R. L., Pharmacologic Treatment of Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern.Med 165(2), 103-112. 5-3-2016.![]()
![]() ; Sateia MJ, Buysse D, Krystal AD, Neubauer DN, Heald JL. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline J Clin Sleep Med 2017;13(2):307-349
; Sateia MJ, Buysse D, Krystal AD, Neubauer DN, Heald JL. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline J Clin Sleep Med 2017;13(2):307-349![]()
![]() ; Riemann D, Baglioni C, Bassetti C et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26(6):675-700
; Riemann D, Baglioni C, Bassetti C et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26(6):675-700![]()
![]() . [return]
. [return]
80. Wyeth v. Levine, 555 U.S. 555 (2009). The full text of the majority opinion by Justice John Paul Stevens, as well as the concurrences and dissent, are available at Cornell University Law School's online Legal Information Institute, at www.law.cornell.edu/supct/html/06-1249.ZS.html![]()
![]() , while a summary of its holdings may be found at Wikipedia, en.wikipedia.org/wiki/Wyeth_v._Levine
, while a summary of its holdings may be found at Wikipedia, en.wikipedia.org/wiki/Wyeth_v._Levine![]()
![]() . [[return]
. [[return]
81. See Wikipedia's summary of the Vioxx case history, as part of its article on Merck and Co., Inc., available online at en.wikipedia.org/wiki/Merck_%26_Co.#Vioxx![]()
![]() . [return]
. [return]
82. Fick DM. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015;63(11):2227-2246. [return]
83. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
84. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
85. Benac N. Tweet That: #covfefe signals @realDonaldTrump is back. AP News. 6-1-2017. Washington, DC, Associated Press.![]()
![]() . [return]
. [return]
86. Kripke, DF. What do hypnotics cost hospitals and healthcare? F1000Res. 6, 542.![]()
![]() 2017. IOM (Institute of Medicine). 2012. [return]
2017. IOM (Institute of Medicine). 2012. [return]
87. PubMed.gov is a free resource that is developed and maintained by the National Center for Biotechnology Information (NCBI), at the U.S. National
Library of Medicine (NLM), located at the National Institutes of Health (NIH).
Available online at www.ncbi.nlm.nih.gov/pubmed![]()
![]() . [return]
. [return]
88. Kripke DF: Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit [version 3]. F1000Res. 2018, 5:918![]()
![]() . [return]
. [return]
89. Wilt, T. J., MacDonald, R., Brasure, M. , Olson, C. M., Carlyle, M., Fuchs, E., Khawaja, I. S., Diem, S., Koffel, E., Ouellette, J., Butler, M., and Kane, R. L., Pharmacologic Treatment of Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern.Med 165(2), 103-112. 5-3-2016.![]()
![]() [return]
[return]
90. More information about Scripps Clinic Viterbi Family Sleep Center in La Jolla, California, is available online,
www.scripps.org/services/sleep-medicine![]()
![]() , or by phoning (858) 554-8845. [[return]
, or by phoning (858) 554-8845. [[return]
91. The American Academy of Sleep Medicine (AASM), a national accrediting body for sleep disorders centers and laboratories, lists more than 2,000 of its accredited centers and labs at its website sleepeducation.org/find-a-facility![]()
![]() , and provides patient education at its website sleepeducation.org
, and provides patient education at its website sleepeducation.org![]()
![]() . [return]
. [return]
Table of Contents
The Dark Side of Sleeping Pills, in all its formats, including this eBook, copyright ©1997-2019 by Daniel F. Kripke, M.D. All rights reserved.